Abstract
Thyromimetics represent a class of experimental drugs that can stimulate tissue-selective thyroid hormone action. As such, thyromimetics should have effects on the hypothalamic-pituitary-thyroid (HPT) axis, but details of this action and the subsequent effects on systemic thyroid hormone levels have not been reported to date. Here, we compare the HPT-axis effects of sobetirome, a well-studied thyromimetic, with Sob-AM2, a newly developed prodrug of sobetirome that targets sobetirome distribution to the central nervous system (CNS). Similar to endogenous thyroid hormone, administration of sobetirome and Sob-AM2 suppress HPT-axis gene transcript levels in a manner that correlates to their specific tissue distribution properties (periphery vs CNS, respectively). Dosing male C57BL/6 mice with sobetirome and Sob-AM2 at concentrations ≥10 μg/kg/d for 29 days induces a state similar to central hypothyroidism characterized by depleted circulating T4 and T3 and normal TSH levels. However, despite the systemic T4 and T3 depletion, the sobetirome- and Sob-AM2-treated mice do not show signs of hypothyroidism, which may result from the presence of the thyromimetic in the thyroid hormone–depleted background.
Thyroid status and HPT-axis effects were evaluated in C57BL/6 mice after they received either a peripherally restricted or a new CNS-penetrating thyromimetic.
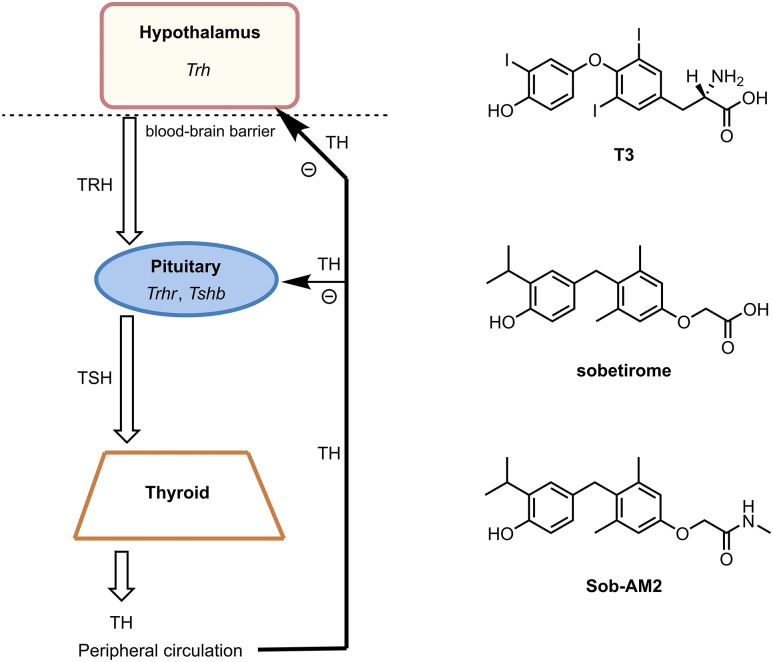
The hypothalamic-pituitary-thyroid (HPT) axis is a multicomponent neuroendocrine system responsible for regulating systemic thyroid hormone levels in higher vertebrates (1–3). Thyroid hormone equilibrium is maintained through a series of feedback loops interfacing at several junctions throughout the neuroendocrine axis (Fig. 1). Signaling about the axis is controlled by three hormones generated within, and secreted from, the three major axis tissues (4). At the top of the axis, the hypothalamus receives cues from thyroid hormone receptor (TR) activation by thyroid hormones that distribute to the brain from circulation via the transporter MCT8 (5). TR activation by T3 in the hypothalamus prompts the suppression of TRH synthesis in the hypothalamic paraventricular nucleus. TRH from the paraventricular nucleus is mobilized through the median eminence and is transported via hypophyseal portal vessels to the anterior pituitary gland, where it binds and activates the TRH receptor. The pituitary senses TRH receptor activation and responds by synthesizing and secreting TSH. In addition, TSH synthesis in the pituitary is regulated by local TR activation from T3 delivered into the gland from peripheral circulation. Transportation of TSH from the pituitary to the thyroid gland and subsequent activation of the TSH receptor drive the synthesis and secretion of thyroid hormone predominantly as T4 into systemic circulation. T4 is deiodinated to the active form of thyroid hormone T3 in a tissue-selective manner. Circulating levels of T3 (and T4 to a lesser extent) dictate the concentration of TRH and TSH produced within the axis to maintain homeostasis (6).
Figure 1.
HPT axis diagram with major feedback loops and the drug molecules used in this study. TH, thyroid hormone.
Certain thyroid hormone actions provide potentially beneficial therapy for a variety of diseases and disorders affecting lipid metabolism (7), obesity (8, 9), glucose metabolism (10), and, more recently, central nervous system (CNS)–related disorders such as multiple sclerosis (11–14). However, thyroid hormone lacks a therapeutic index separating systemic thyrotoxicity from beneficial therapeutic effects. Sobetirome is a potent, clinical-stage thyroid hormone receptor beta isoform-selective thyromimetic with a useful therapeutic index compared with thyroid hormone (15, 16). In an ongoing effort to develop approaches to increase sobetirome exposure in the CNS, we recently discovered an efficient CNS-penetrating prodrug of sobetirome called Sob-AM2. A peripheral dose of Sob-AM2 delivers about 10-fold more sobetirome to the CNS compared with unmodified sobetirome while masking sobetirome while it resides in systemic circulation (17–19). Sob-AM2 therefore serves as a probe for investigating local thyroid hormone action within the CNS. Moreover, the combination of sobetirome and Sob-AM2 provide tools to probe the HPT-axis effects that result from predominant peripheral and central T3 agonism, respectively. In this study, we compare and contrast what effects sobetirome and Sob-AM2 have on the HPT-axis signaling cascade, thyroid status, and biometrics of wild-type C57BL/6 mice.
Material and Methods
Reagents
Sobetirome (17) and Sob-AM2 (19) were prepared as described in the literature. T3 was purchased and used as received from Sigma-Aldrich. All drugs were prepared at concentrations suitable for an IP injection of 150 μL per 26-g mouse. Sobetirome and Sob-AM2 drug stocks were prepared in 50% dimethyl sulfoxide (DMSO) in saline solutions; T3 drug stocks were prepared in 8 mM NaOH in saline. Vehicle stock solutions of 50% DMSO in saline (vehicle for sobetirome and Sob-AM2) or 8 mM NaOH in saline (T3 vehicle) were prepared and administered within the appropriate experiments.
Mouse experiments
Experimental protocols were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. Wild-type C57BL/6 mice, aged 8 to 10 weeks, were purchased from Jackson Laboratory and housed in climate-controlled rooms with a 12-hour light/12-hour dark cycle with ad libitum access to food and water. All injections were delivered IP using 1:1 saline/DMSO as vehicle for sobetirome and Sob-AM2 or 8 mM NaOH in saline for T3, corresponding to a standardized volume of 150 μL per 26-g mouse. Euthanasia was carried out with CO2 followed by cervical dislocation.
Axis gene suppression data from quantitative PCR was obtained from tissues collected 6 hours after a single administration of T3, sobetirome, or Sob-AM2 to hypothyroid C57BL/6 mice over a dose range with n = 5 per dose per compound. Administered dose ranges were as follows: T3 = 0.305, 0.0914, 0.0305, 0.00914, 0.000914, and 0.0000914 μmol/kg; sobetirome = 30.5, 3.05, 0.305, 0.0914, 0.00914, and 0.000914 μmol/kg; and Sob-AM2 = 30.5, 3.05, 0.305, 0.0914, 0.00914, and 0.000914 μmol/kg. For reference, a sobetirome dose of 3.05 μmol/kg is equivalent to 1 mg/kg sobetirome. Mice were made hypothyroid by receiving 0.1% (w/v) methimazole and 0.2% (w/v) potassium perchlorate (Sigma-Aldrich) in drinking water for 2 weeks (20). Hypothalami and pituitary glands were microdissected and/or removed according to generous guidance provided by staff in the Department of Comparative Medicine at the Oregon Health and Science University. Tissues were immediately preserved in RNAlater (Thermo Fisher Scientific, Waltham, MA) for RNA transcript analysis.
In the chronic-dosing experiment, euthyroid, wild-type C57BL/6 mice received a once-daily injection of either sobetirome or Sob-AM2 at 100, 10, or 1 μg/kg with n = 4 per dose per compound for 29 days. For reference, these doses correspond to 0.305, 0.0305, and 0.00305 μmol/kg sobetirome, respectively. Whole blood was collected 6 hours after the final dose, incubated for at least 15 minutes on ice, then spun at 7800 rpm for 15 minutes at 4°C to isolate serum, which was stored at −80°C until further processing for total T4 and TSH measurements. To validate a reference range for TSH measurements, a cohort of mice was rendered hypothyroid by receiving 0.1% (w/v) methimazole and 0.2% (w/v) potassium perchlorate in drinking water for 2 weeks. An additional reference cohort of hyperthyroid mice was generated by administration of 1 mg/kg T3 daily for 5 days; serum was collected 6 hours after receiving the final dose.
Quantitative PCR
Hypothalamus and pituitary RNA was purified from RNA later-preserved tissue according to a protocol for RNA extraction with TRIzol reagent and the PureLink RNA mini kit (Life Technologies), using a Qiagen RNase-free DNase kit during the optional DNase treatment step. RNA was quantified using a Nanodrop (Thermo Fisher Scientific), and extracted RNA (1 μg) was used to synthesize cDNA via an RT reaction using the QuantiTect Reverse Transcription kit (Qiagen). DNA contamination was controlled for by duplicating one sample without the addition of RT. Quantitative PCR was performed on an Applied Biosciences 7500 Real-Time PCR system following the QuantiTect SYBR Green PCR kit protocols (Qiagen). For all samples, cyclophilin A (f-Ppia: 5′-AGGGTGGTGACTTTACACGC-3′; r-Ppia: 5′-CTTGCCATCCAGCCATTCAG-3′) was used as the housekeeping gene for normalizing between samples. Transcript levels of Trh in hypothalami were measured using the following primers (f-Trh: 5′-GGTGCTGCCTTAGATTCCTG-3′; r-Trh: 5′-CTTGTCTTGGTTGGCACGTC-3′). In the pituitary, Tshb transcripts were measured using the final set of primers (f-Tshb: 5′-GAGAGTGGGTCATCACAGCAG-3′; r-Tshb: 5′-GCAGCACTCATGCTTTGAAC-3′). Data analysis was performed using the comparative 2-ΔΔCt method to monitor the relative differences in gene expression (21). Individual data points are represented as normalized fold-change ± SEM; a sigmoidal dose-response model in GraphPad Prism 7 (GraphPad Software) was then used to generate ED50 values from nonlinear regression. Experiments were performed with technical duplicates.
Radioimmunoassays
Total T4 was measured in mouse sera (25 μL per individual sample) following the manufacturer’s instructions, using the Vet Total T4 Coated Tube RIA Test Kit (IVD Technologies, TT4-1000V-100/500). Standards for the T4 assay ranged from 0.3 to 10 μg/dL. Similarly, total T3 was analyzed in mouse sera (100 μL per individual sample) following the manufacturer’s instructions, using the Vet Total T3 Coated Tube RIA Test Kit (IVD Technologies; TT3-1000V-100/500). Results are expressed as means ± SEM. TSH was measured in mouse sera (100 μL per individual sample) following the manufacturer’s instructions, using the Rat TSH [125I] RIA Kit (MP Biomedicals, LLC; catalog no. 07RK-554). Standards for the TSH assay ranged from 1 to 64 ng/mL. All experimental values fell within a range found to correlate with those previously published (22, 23). Results are expressed as means ± SEM.
Statistical methods
Statistical analyses were performed using the two-tailed Student t test comparing individual groups with the appropriate vehicle group or as noted. Significance level was set to <0.05 with P values illustrated with the following symbols: ns = not significant, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. All data represent mean ± SEM. Animal group numbers were informed by previous work to minimize total animal numbers as appropriate per experiment. Data were plotted and analyzed using GraphPad Prism 7.
Results
Acute response: transcription suppression of axis signaling genes in vivo
To assess how thyromimetics affect HPT-axis signaling, hypothalamic Trh and pituitary Tshb mRNA levels were measured by quantitative PCR from tissues of treated mice to produce dose-response curves for each gene (Fig. 2). Age-matched, wild-type C57BL/6 male mice were made hypothyroid via ad libitum drinking water treatment with methimazole (0.2%) and potassium perchlorate (0.1%) for 14 days (20) to increase the dynamic range of the changes to Trh and Tshb transcripts upon thyromimetic treatment. Mice received T3, sobetirome, Sob-AM2 over a dose range (30.5 to 0.0000914 μmol/kg), or vehicle (IP); tissues were collected 6 hours postinjection. Quantification of mRNA from hypothalamic tissue revealed the following trend in potency for Trh suppression: T3 > Sob-AM2 > sobetirome. T3 administration induced the greatest degree of suppression for each axis gene studied (24, 25). Of the thyromimetics, CNS-penetrating Sob-AM2 (ED50 = 0.11 μmol/kg) was approximately an order of magnitude more potent at Trh downregulation compared with sobetirome (ED50 = 1.4 μmol/kg) (Fig. 2A). However, the trend was reversed in the case of pituitary Tshb activation in which sobetirome (ED50 = 0.088 μmol/kg) was found to be 3.2-fold more potent at activating expression of this gene compared with Sob-AM2 (ED50 = 0.28 μmol/kg) (Fig. 2C).
Figure 2.
Quantitative PCR dose-response curves for axis gene suppression at 6 h following administration of T3, sobetirome, or Sob-AM2. Samples were run in duplicate with n = 5 per dose per compound. All data points represent mean ± SEM.
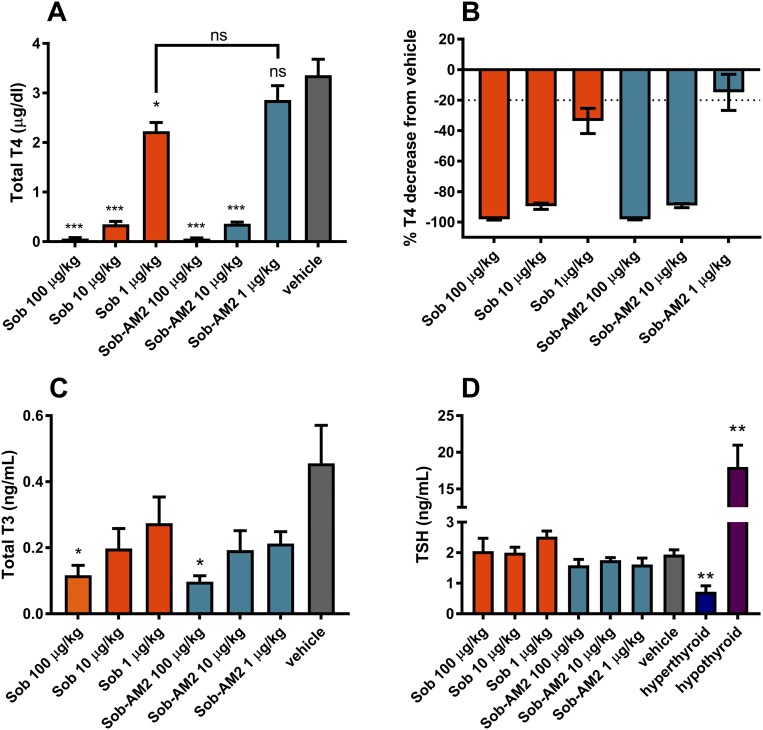
Thyroid status from chronic dosing
The observed transcriptional suppression of Tshb by thyromimetics suggests circulating TSH suppression and subsequent depletion of circulating thyroid hormone caused by these agents. To assess the terminal effect of axis suppression, thyroid status was measured in mice after long-term daily dosing with the thyromimetics. Euthyroid wild-type mice were treated with vehicle, sobetirome, or Sob-AM2 (IP) once daily for 29 days over a dose range spanning two orders of magnitude: 1 μg/kg (∼0.003 μmol/kg), 10 μg/kg (∼0.03 μmol/kg), or 100 μg/kg (∼0.3 μmol/kg). This dose range was selected on the basis of the gene activation data described previously, suggesting that the sensitive portion of the dose-response curve would reside within this range. Serum total T4, T3, and TSH were measured 6 hours postinjection on the final day of treatment (Fig. 3). Full serum depletion of T4 was observed at the high dose of 100 μg/kg for both sobetirome and Sob-AM2 (Fig. 3A and 3B). Nearly full depletion (>90%) occurred for both compounds at 10 μg/kg, whereas a small, partial depletion was observed for sobetirome (34%) at 1 μg/kg, and serum T4 was not significantly affected vs vehicle by 1 μg/kg Sob-AM2 (Fig. 3A and 3B). Total serum T3 levels mirrored the T4 results, with T3 depletion occurring in response to escalating dose for both sobetirome and Sob-AM2. Serum T3 concentrations at the 1- and 10-μg/kg doses of sobetirome and Sob-AM2 were not significantly different from vehicle, whereas both compounds at 100 μg/kg doses affected significant T3 lowering compared with vehicle (Fig. 3C). In contrast to these depleted circulating T4 and T3 levels and suppression of Tshb from acute dosing, serum TSH levels were found to be in the normal range for all doses of sobetirome and Sob-AM2 (Fig. 3D).
Figure 3.
(A, B) Total T4, (C) total T3, and (D) TSH measurements in systemic circulation after chronic treatment with Sob and Sob-AM2 IP for 29 d (n = 4 per group). (D) Hyperthyroid mice (n = 5) were given 1 mg/kg T3 daily for 5 d; hypothyroid mice (n = 5) were administered methimazole and perchlorate water for 2 wk. All data represent mean ± SEM. Statistical analyses were performed using the two-tailed Student t test comparing individual groups with the appropriate vehicle group or as noted. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. ns, not significant; sob, sobetirome.
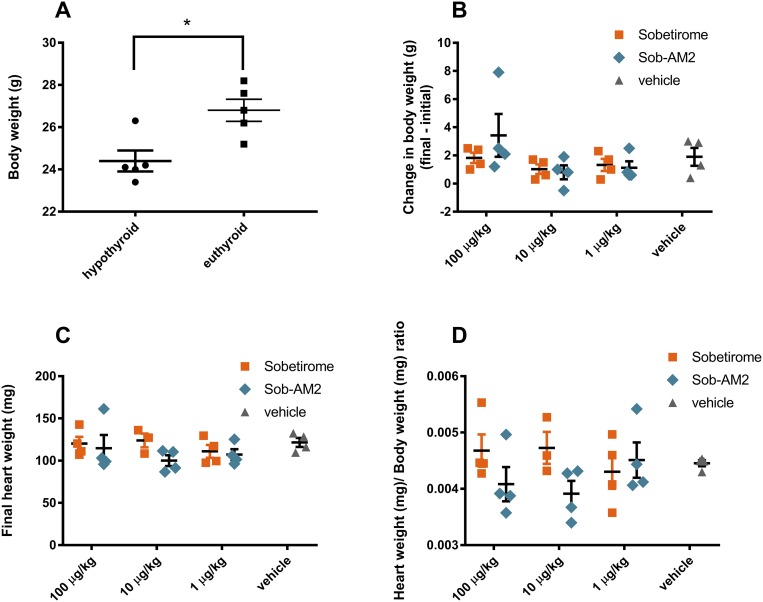
Body and heart weight effects
Body weight measurements were compared between age-matched euthyroid and hypothyroid (see the previous section) control cohorts, and the hypothyroid mice were found to have reduced body weight compared with euthyroid mice, consistent with previous reports (Fig. 4A) (26). Initial and final body weights were measured for each sobetirome and Sob-AM2 dose group in the 29-day once-daily dosing experiment, and the difference (final – initial) in body weight per mouse was determined (Fig. 4B). There was no significant change to body weight found between any group receiving a thyromimetic and vehicle. Similarly, there were no significant differences between vehicle and treatment groups comparing final heart weights (Fig. 4C), or heart weight to body weight ratio (Fig. 4D). Pooling of the 100- and 10-μg/kg data for sobetirome and Sob-AM2 also failed to produce significant differences compared with vehicle (data not shown). Additionally, at no point during the 29-day experiment did the appearance or behavior of thyromimetic-treated mice differ from that of vehicle control mice.
Figure 4.
(A) Differences in body weight between age-matched hypothyroid and euthyroid mice. (B–D) Heart and body weight comparisons after chronic IP dosing with sobetirome and Sob-AM2 for 29 d. All data represent mean ± SEM. Statistical analyses were performed using the two-tailed Student t test comparing individual groups with the appropriate vehicle group or as noted. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Discussion and Conclusion
Selective thyroid hormone agonists, or thyromimetics, are promising experimental therapeutics for a number of potential indications. The tissue selectivity of these agents enables thyroid hormone agonist actions in tissues that produce a beneficial effect from excessive thyroid hormone signaling. For thyromimetics to be clinically useful, they should have reduced thyroid agonist actions in tissues in which excessive thyroid hormone signaling produces adverse effects, most notably in heart, bone, and skeletal muscle. Sobetirome is a clinical-stage thyromimetic that meets these basic criteria (15). Its selective T3 actions in animal models suggest clinical uses in hyperlipidemia (26), obesity (27), and fibrosis (28) while also being devoid of the adverse effects on heart (26, 29), bone (30), and skeletal muscle (31) normally associated with hyperthyroidism. Sobetirome is also the only thyromimetic agent reported to date to penetrate the blood-brain barrier and distribute to the CNS from a systemic dose administration (17, 26, 32). This opens up the possibility of studying sobetirome in CNS disease models that may illuminate benefits of selective thyroid hormone action in the CNS. Multiple sclerosis (33), X-linked adrenoleukodystrophy (14), and MCT8 deficiency (Allan-Herndon-Dudley syndrome) (34) are examples of such CNS diseases in which sobetirome has shown promise in relevant models. These applications in the CNS have led to the development of prodrugs of sobetirome that deliver greater amounts of sobetirome to the CNS from a systemic dose while also minimizing the peripheral sobetirome exposure (17–19). Sob-AM2 is a particularly effective sobetirome prodrug in this regard because it increases sobetirome CNS exposure by almost 10-fold and increases distribution from systemic circulation to the brain (brain/serum ratio) by 60-fold compared with systemic dosing of unmodified sobetirome (19).
As would be expected for a TRβ-selective T3 agonist, sobetirome has dose-dependent actions that affect the HPT axis. In rats treated once daily for 7 days, sobetirome suppressed circulating TSH in a dose-dependent fashion, although less potently than TSH suppression by T3 in the rat (26). Although substantial TSH suppression was observed, circulating T4 was only moderately reduced (37% vs control) after 7 days of dosing with sobetirome in rats, suggesting that equilibrium had not yet been established within the HPT axis with a 7-day, once-daily sobetirome dosing schedule (29). Partial T4 depletion has also been observed in mice after 7 days of once-daily sobetirome (34). Because many of the CNS disease models discussed here require longer than 7-day dosing periods, we wanted to understand the HPT-axis effects of sobetirome under conditions of longer exposure. In addition, we wanted to assess any differences in HPT-axis effects between dosing with sobetirome, which largely confines sobetirome exposure to the periphery, and Sob-AM2, which largely confines sobetirome exposure to the CNS.
Here we report that this difference in peripheral and central exposure of sobetirome arising from whether animals are dosed with Sob-AM2 or unmodified sobetirome correlates with observed potency of repression of hypothalamic Trh and pituitary Tshb (Fig. 2; Table 1). A systemic dose of Sob-AM2, which delivers about 10-fold more sobetirome to the brain compared with unmodified sobetirome, was about 10-fold more potent than sobetirome at suppressing expression of Trh in the hypothalamus. In contrast, sobetirome was found to be more potent (threefold) than Sob-AM2 at suppressing expression of Tshb in the pituitary. T3 was found to be substantially more potent than either sobetirome or Sob-AM2 at suppressing all three genes regardless of location. Accordingly, the higher concentrations of sobetirome in the brain and lower concentrations in the blood afforded by dosing with Sob-AM2 stimulates to a greater degree the T3 agonist axis genes that reside in the brain. Conversely, the higher blood levels of sobetirome arising from sobetirome vs Sob-AM2 dosing results in more effective suppression of Tshb, the pituitary axis gene that directly results in TSH expression. The net effect of suppression of these HPT axis genes is to suppress TSH secretion from the pituitary, resulting ultimately in systemic thyroid hormone depletion. As mentioned previously, substantial T4 depletion was not previously observed from 7 days of dosing with comparable doses of sobetirome to those used in this study, even though robust TSH suppression was observed. However, we find that extending the dosing duration from 7 to 29 days results in dose-dependent T4 and T3 depletion, fully depleting circulating T4 at a daily sobetirome dose between 10 and 100 μg/kg/day (Fig. 3A and 3B). Less potent dose-dependent lowering of serum T3 is observed, likely in part to peripheral deiodination of T4 in response to HPT-axis suppression. The potency of T4 depletion is comparable between sobetirome and Sob-AM2, with sobetirome being moderately more potent than Sob-AM2. This indicates that the indirect hypothalamic vs pituitary gene suppression selectivity displayed by Sob-AM2 plays a lesser role in TSH secretion from the pituitary compared with direct pituitary suppression of TSH. In contrast to the marked T4 depletion, circulating TSH levels were found to be normal after 29 days of dosing with either sobetirome or Sob-AM2 (Fig. 3D).
Table 1.
ED50 Values Derived From the Dose-Response Experiments in Fig. 2
| Compound | Trh ED50 (μmol/kg) ± SE | Tshb ED50 (μmol/kg) ± SE |
|---|---|---|
| T3 | 0.00093 ± 0.0041 | 0.0042 ± 0.015 |
| Sobetirome | 1.4 ± 1.9 | 0.088 ± 0.044 |
| Sob-AM2 | 0.11 ± 0.34 | 0.28 ± 0.38 |
T4 depletion in the presence of normal TSH levels is diagnostic of a condition called “central hypothyroidism.” Often caused by lesions such as a tumor affecting the function of the hypothalamus or pituitary, central hypothyroidism is usually diagnosed based on biochemical findings of low circulating T4 combined with normal or low/normal TSH (35). Treatment of central hypothyroidism is similar to that of primary hypothyroidism wherein circulating thyroid hormone levels are restored with hormone replacement therapy. Drugs that suppress TSH such as the rexinoid bexarotene can also lead to central hypothyroidism; thyroid function typically returns to normal once the drug is discontinued, illustrating that this drug-induced central hypothyroidism results solely from HPT-axis suppression and not damage to the thyroid gland (36). Bexarotene suppresses TSH secretion indirectly through suppression of hypothalamic TRH; bexarotene does not exert any direct suppressive effects on the pituitary (37). That sobetirome was previously shown to suppress TSH at sufficiently high doses in a 7-day dosing regimen and that, after 29 days at this dose, T4 is substantially depleted, with TSH returning to normal as shown in this study, suggests that the competition between sobetirome T3 agonism and low endogenous thyroid hormones that occurs at the hypothalamus and pituitary leads to an inappropriate TSH response to the T4 depletion. This inappropriate TSH response to depleted circulating thyroid hormones is a thyroid function test hallmark of central hypothyroidism, thus distinguishing it from primary hypothyroidism in which the defect in the HPT axis resides with the thyroid gland. Iatrogenic sources of central hypothyroidism are speculated to mechanistically involve the production of sialylated, bioinactive TSH (38–40). It has also been suggested that normal or high TSH levels in central hypothyroidism can be justified by alterations in signaling networks adjacent to the HPT axis; hypoadrenalism and diminished somatostatin secretion from the hypothalamus boost TSH production (38, 41, 42). These mechanistic details cannot be strictly ruled out here.
There were no signs of hypothyroidism observed in this 29-day study despite the substantial T4 depletion that occurred at 10 and 100 μg/kg/day and T3 depletion at 100 μg/kg/day with both sobetirome and Sob-AM2. Mice rendered hypothyroid with treated water (see “Materials and Methods”) for 29 days lose significant body weight compared with euthyroid controls (Fig. 4A); no changes in body weight, heart weight, or heart/body weight ratio were observed in any of the sobetirome- or Sob-AM2-treated mice (Fig. 4B and 4D). We have reported previously that a longer duration treatment of 90 days with larger sobetirome doses of 400 to 2000 μg/kg/day can produce alopecia (hair loss), body weight loss of up to 20%, and death (14). Mice treated for 90 days with 80 μg/kg/day sobetirome, a dose that would result in substantial T4 depletion based on the results presented here, exhibited none of these symptoms. Chronic hypothyroidism in mice is known to produce alopecia, body weight loss, and premature death (26, 43, 44). This indicates that the adverse effects in mice that occurred with sobetirome doses >400 μg/kg/day in the 90-day study may have been a result of the indirect pharmacological action of sobetirome-induced T4 depletion and not as a direct toxicological action of sobetirome acting as a T3 agonist on a tissue other than the HPT axis. That these hypothyroid-like adverse effects are dose dependent within a dose range that creates a T4- and T3-depleted background suggests that at least a subset of the T3-agonist effects of sobetirome are compensating for the depleted endogenous thyroid hormones.
In summary, sobetirome and its CNS-targeting prodrug Sob-AM2 produce a state of circulating thyroid hormone depletion in mice treated with doses of ≥10 μg/kg/d for ≥29 days. It is likely that other thyromimetic agents in use have a similar action. Most animal model studies using these agents reported to date are designed to report on tissue selective T3 agonist actions that typically occur more rapidly than the time of daily exposure required to reset the HPT axis to produce the systemic T4 depletion documented here. However, for studies involving daily treatment of 29 days or longer at similar or larger doses as those used here, thyroid hormone depletion will occur and should be taken into account in interpretation of the data. For example, T3-treated mice are often used as a control group in studies using sobetirome, and the results presented here suggest that there are complexities to this comparison. It should now be understood that this is not a simple comparison between treatment with a selective T3 agonist (sobetirome) and a nonselective agonist (T3). Rather, it is a comparison between a hyperthyroid (T3-treated) cohort and a cohort that is thyroid hormone–depleted (sobetirome-treated) with a selective T3 agonist applied over the top of this background. It will be instructive to determine any changes that occur in sobetirome studies of ≥29 days’ duration in which the sobetirome-induced T4 depletion is corrected by thyroid hormone replacement.
Acknowledgments
We thank the staff of the Department of Comparative Medicine at the Oregon Health and Science University for technical assistance. The authors also thank Tania Banerji, Ilsa Kirby, and Dr. Meredith Hartley for helpful discussions.
Financial Support: This research was supported by the Oregon Health and Science University Laura Fund for Innovation in Multiple Sclerosis, National Institutes of Health Grant DK52798 (to T.S.S.), National Multiple Sclerosis Society Grant RG5199A4/1 (to D.B.), and Race to Erase MS (to D.B.).
Disclosure Summary: T.S.S. and S.J.F. are inventors on a pending patent application claiming central nervous system–penetrating prodrugs of sobetirome and their uses. T.S.S. is a founder of NeuroVia, Inc. The remaining author has nothing to disclose.
Glossary
Abbreviations:
- CNS
central nervous system
- DMSO
dimethyl sulfoxide
- HPT
hypothalamic-pituitary-thyroid
- TR
thyroid hormone receptor
References
- 1. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fliers E, Kalsbeek A, Boelen A. Beyond the fixed setpoint of the hypothalamus-pituitary-thyroid axis. Eur J Endocrinol. 2014;171(5):R197–R208. [DOI] [PubMed] [Google Scholar]
- 3. Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37(1-2):11–53. [DOI] [PubMed] [Google Scholar]
- 4. Medici M, Visser WE, Visser TJ, Peeters RP. Genetic determination of the hypothalamic-pituitary-thyroid axis: where do we stand? Endocr Rev. 2015;36(2):214–244. [DOI] [PubMed] [Google Scholar]
- 5. Bernal J, Guadaño-Ferraz A, Morte B. Thyroid hormone transporters-functions and clinical implications [published corrections appear in Nat Rev Endocrinol. 2015;11:506 and 2015;11:690] Nat Rev Endocrinol. 2015;11(12):406–417. [DOI] [PubMed] [Google Scholar]
- 6. Chiamolera MI, Wondisford FE. Minireview: thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150(3):1091–1096. [DOI] [PubMed] [Google Scholar]
- 7. Müller MJ, Acheson KJ, Jequier E, Burger AG. Thyroid hormone action on lipid metabolism in humans: a role for endogenous insulin. Metabolism. 1990;39(5):480–485. [DOI] [PubMed] [Google Scholar]
- 8. Krotkiewski M. Thyroid hormones in the pathogenesis and treatment of obesity. Eur J Pharmacol. 2002;440(2-3):85–98. [DOI] [PubMed] [Google Scholar]
- 9. Sanyal D, Raychaudhuri M. Hypothyroidism and obesity: an intriguing link. Indian J Endocrinol Metab. 2016;20(4):554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci USA. 2009;106(14):5966–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez M, Giuliani A, Pirondi S, D’Intino G, Giardino L, Aloe L, Levi-Montalcini R, Calzà L. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc Natl Acad Sci USA. 2004;101(46):16363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calzà L, Fernandez M, Giardino L. Cellular approaches to central nervous system remyelination stimulation: thyroid hormone to promote myelin repair via endogenous stem and precursor cells. J Mol Endocrinol. 2010;44(1):13–23. [DOI] [PubMed] [Google Scholar]
- 13. Zhang M, Ma Z, Qin H, Yao Z. Thyroid hormone potentially benefits multiple sclerosis via facilitating remyelination. Mol Neurobiol. 2016;53(7):4406–4416. [DOI] [PubMed] [Google Scholar]
- 14. Hartley MD, Kirkemo LL, Banerji T, Scanlan TS. A thyroid hormone-based strategy for correcting the biochemical abnormality in X-linked adrenoleukodystrophy. Endocrinology. 2017;158(5):1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scanlan TS. Sobetirome: a case history of bench-to-clinic drug discovery and development. Heart Fail Rev. 2010;15(2):177–182. [DOI] [PubMed] [Google Scholar]
- 16. Lammel Lindemann J, Webb P. Sobetirome: the past, present and questions about the future. Expert Opin Ther Targets. 2016;20(2):145–149. [DOI] [PubMed] [Google Scholar]
- 17. Placzek AT, Ferrara SJ, Hartley MD, Sanford-Crane HS, Meinig JM, Scanlan TS. Sobetirome prodrug esters with enhanced blood-brain barrier permeability. Bioorg Med Chem. 2016;24(22):5842–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrara SJ, Meinig JM, Placzek AT, Banerji T, McTigue P, Hartley MD, Sanford-Crane HS, Banerji T, Bourdette D, Scanlan TS. Ester-to-amide rearrangement of ethanolamine-derived prodrugs of sobetirome with increased blood-brain barrier penetration. Bioorg Med Chem. 2017;25(10):2743–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meinig JM, Ferrara SJ, Banerji T, Banerji T, Sanford-Crane HS, Bourdette D, Scanlan TS. Targeting fatty-acid amide hydrolase with prodrugs for CNS-selective therapy. ACS Chem Neurosci. 2017;8(11):2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hackenmueller SA, Marchini M, Saba A, Zucchi R, Scanlan TS. Biosynthesis of 3-iodothyronamine (T1AM) is dependent on the sodium-iodide symporter and thyroperoxidase but does not involve extrathyroidal metabolism of T4. Endocrinology. 2012;153(11):5659–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manzano J, Morte B, Scanlan TS, Bernal J. Differential effects of triiodothyronine and the thyroid hormone receptor beta-specific agonist GC-1 on thyroid hormone target genes in the b ain. Endocrinology. 2003;144(12):5480–5487. [DOI] [PubMed] [Google Scholar]
- 23. Fonseca TL, Correa-Medina M, Campos MP, Wittmann G, Werneck-de-Castro JP, Arrojo e Drigo R, Mora-Garzon M, Ueta CB, Caicedo A, Fekete C, Gereben B, Lechan RM, Bianco AC. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest. 2013;123(4):1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schomburg L, Bauer K. Thyroid hormones rapidly and stringently regulate the messenger RNA levels of the thyrotropin-releasing hormone (TRH) receptor and the TRH-degrading ectoenzyme. Endocrinology. 1995;136(8):3480–3485. [DOI] [PubMed] [Google Scholar]
- 25. Shupnik MA, Ridgway EC. Thyroid hormone control of thyrotropin gene expression in rat anterior pituitary cells. Endocrinology. 1987;121(2):619–624. [DOI] [PubMed] [Google Scholar]
- 26. Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141(9):3057–3064. [DOI] [PubMed] [Google Scholar]
- 27. Lin JZ, Martagón AJ, Cimini SL, Gonzalez DD, Tinkey DW, Biter A, Baxter JD, Webb P, Gustafsson JÅ, Hartig SM, Phillips KJ. Pharmacological activation of thyroid hormone receptors elicits a functional conversion of white to brown fat. Cell Reports. 2015;13(8):1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JPW, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo E Drigo R, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018;24(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-L-thyronine. Endocrinology. 2004;145(4):1656–1661. [DOI] [PubMed] [Google Scholar]
- 30. Freitas FRS, Moriscot AS, Jorgetti V, Soares AG, Passarelli M, Scanlan TS, Brent GA, Bianco AC, Gouveia CH. Spared bone mass in rats treated with thyroid hormone receptor TR beta-selective compound GC-1. Am J Physiol Endocrinol Metab. 2003;285(5):E1135–E1141. [DOI] [PubMed] [Google Scholar]
- 31. Miyabara EH, Aoki MS, Soares AG, Saltao RM, Vilicev CM, Passarelli M, Scanlan TS, Gouveia CH, Moriscot AS. Thyroid hormone receptor-beta-selective agonist GC-24 spares skeletal muscle type I to II fiber shift. Cell Tissue Res. 2005;321(2):233–241. [DOI] [PubMed] [Google Scholar]
- 32. Takahashi N, Asano Y, Maeda K, Watanabe N. In vivo evaluation of 1-benzyl-4-aminoindole-based thyroid hormone receptor β agonists: importance of liver selectivity in drug discovery. Biol Pharm Bull. 2014;37(7):1103–1108. [DOI] [PubMed] [Google Scholar]
- 33. Baxi EG, Schott JT, Fairchild AN, Kirby LA, Karani R, Uapinyoying P, Pardo-Villamizar C, Rothstein JR, Bergles DE, Calabresi PA. A selective thyroid hormone β receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia. 2014;62(9):1513–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bárez-López S, Hartley MD, Grijota-Martínez C, Scanlan TS, Guadaño-Ferraz A. Sobetirome and its amide prodrug Sob-AM2 exert thyromimetic action in Mct8-deficient brain. Thyroid (in press). [DOI] [PMC free article] [PubMed]
- 35. Persani L. Clinical review: central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97(9):3068–3078. [DOI] [PubMed] [Google Scholar]
- 36. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2009;23(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu S, Ogilvie KM, Klausing K, Lawson MA, Jolley D, Li D, Bilakovics J, Pascual B, Hein N, Urcan M, Leibowitz MD. Mechanism of selective retinoid X receptor agonist-induced hypothyroidism in the rat. Endocrinology. 2002;143(8):2880–2885. [DOI] [PubMed] [Google Scholar]
- 38. Yamada M, Mori M. Mechanisms related to the pathophysiology and management of central hypothyroidism. Nat Clin Pract Endocrinol Metab. 2008;4(12):683–694. [DOI] [PubMed] [Google Scholar]
- 39. Persani L, Ferretti E, Borgato S, Faglia G, Beck-Peccoz P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J Clin Endocrinol Metab. 2000;85(10):3631–3635. [DOI] [PubMed] [Google Scholar]
- 40. Estrada JM, Soldin D, Buckey TM, Burman KD, Soldin OP. Thyrotropin isoforms: implications for thyrotropin analysis and clinical practice. Thyroid. 2014;24(3):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samuels MH. Effects of metyrapone administration on thyrotropin secretion in healthy subjects--a clinical research center study. J Clin Endocrinol Metab. 2000;85(9):3049–3052. [DOI] [PubMed] [Google Scholar]
- 42. Takano K, Ajima M, Teramoto A, Hata K, Yamashita N. Mechanisms of action of somatostatin on human TSH-secreting adenoma cells. Am J Physiol. 1995;268(4 Pt 1):E558–E564. [DOI] [PubMed] [Google Scholar]
- 43. Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3(3):211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hutchens S, Liu C, Jursa T, Shawlot W, Chaffee BK, Yin W, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J Biol Chem. 2017;292(23):9760–9773. [DOI] [PMC free article] [PubMed] [Google Scholar]