Figure 1:
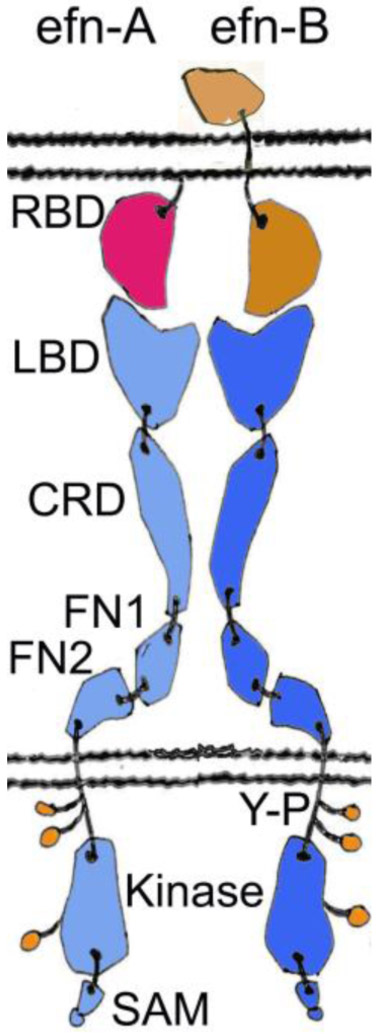
Schematic representation of the Eph receptors and their ephrin (efn) ligands. Ephrin-A is shown in red, ephrin-B in light brown, EphA receptor in cyan, and EphB receptor in blue. The A class ligands are attached to the cell membrane by a glycosylphosphatidylinositol (GPI) linkage and bind nearly exclusively the A class receptors. On the other hand, the B ligands that bind almost exclusively the B class receptors, contain a transmembrane stretch and a small cytoplasmic domain. The overall structures of the EphA and the EphB receptors are very similar. The ectodomain forms a fairly rigid, rod-like structure that undergoes minimal conformational change upon activation. Ligand binding causes a clustering of the Eph receptors, leading to the opening of an activation loop, and a subsequent tyrosine phosphorylation (shown in green) of the kinase domain. SAM domain offers a docking surface for downstream signaling and is reported to be involved in receptor oligomerization (Shi et al., 2017). RBD, Receptor-Binding Domain; LBD, Ligand-Binding Domain; CRD, Cysteine-Rich Domain; FN, Fibronectin III domain; Y-P, Phospho-Tyrosine; SAM, Sterile Alpha Motif.
