Introduction
The prevalence of obesity continues to rise in the United States and impacts on morbidity, mortality and overall costs in health care considerably. In 2013–2014, more than one-third (37.9%) of U.S. adults were classified as obese, and the prevalence of those with a body mass index (BMI) ≥40kg/m2, was 7.7%. (1) The heaviest groups (BMI ≥50kg/m2) increases in prevalence at the fastest rates. (2) Pertinent to healthcare providers—the larger the patient, the more health care resources utilized and the worse the survival. (3) Having a BMI >40kg/m2 predicts use of more than double the healthcare dollars of a normal weight individual. (4)
Providing care to individuals with severe obesity is complex, as the physiologic changes associated with morbid and super obesity alter the pharmacokinetic properties of most drugs. (5) Derangements in cardiovascular and respiratory physiology make the patients with morbid obesity more vulnerable to drug-induced respiratory depression and upper airway obstruction, thus increasing the risk of treating them with opioids. In reviewing the literature, it appears that much of what is understood regarding the care of patients with super obesity must be extrapolated from what is known regarding the morbidly obese, a group that has been studied more frequently. The term “super obesity” has been used variably, but defined often as weighing 150 pounds or more than ideal body weight or having a BMI ≥50kg/m. (6)
Herein we present a case of a patient with a BMI of 100kg/m2 admitted with uncontrolled pain due to large necrotic skin ulcers from nonuremic calciphylaxis. Through this case, we review the limitations of data regarding pharmacologic properties for those with super obesity, and provide palliative medicine providers with a framework for such management.
Case Description
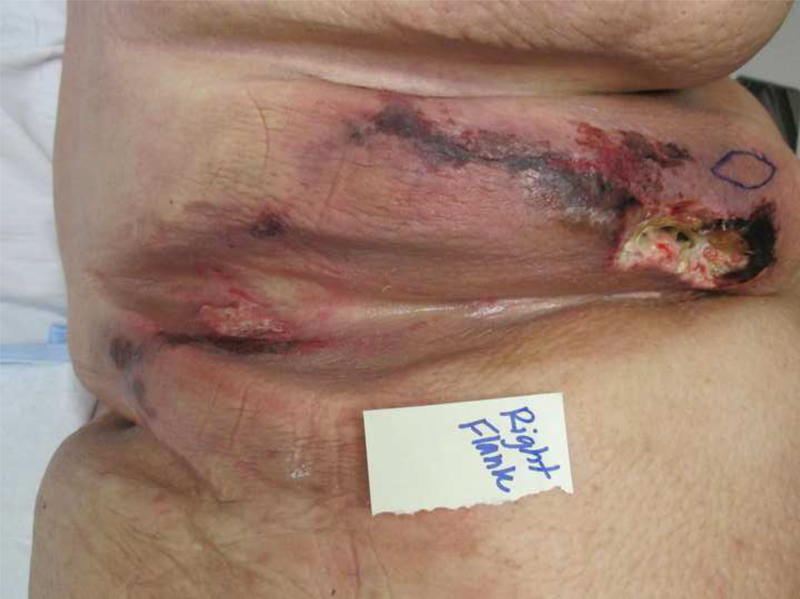
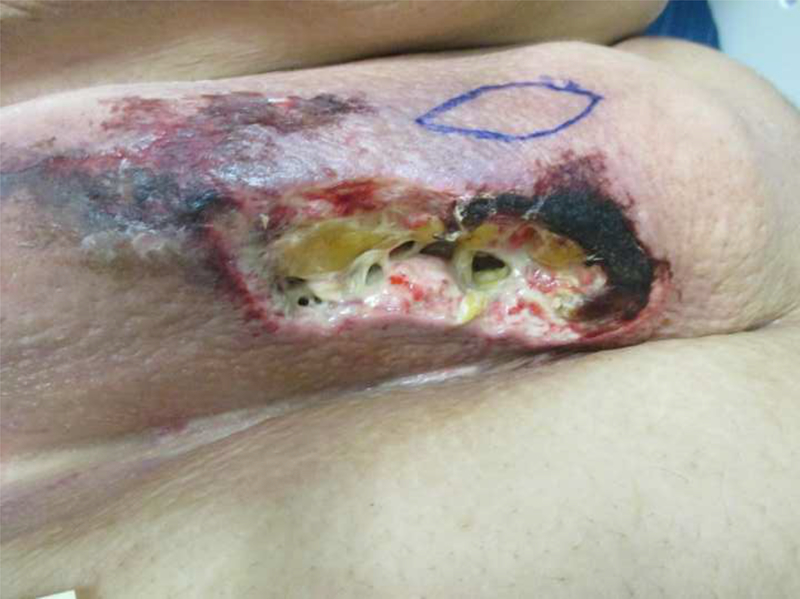
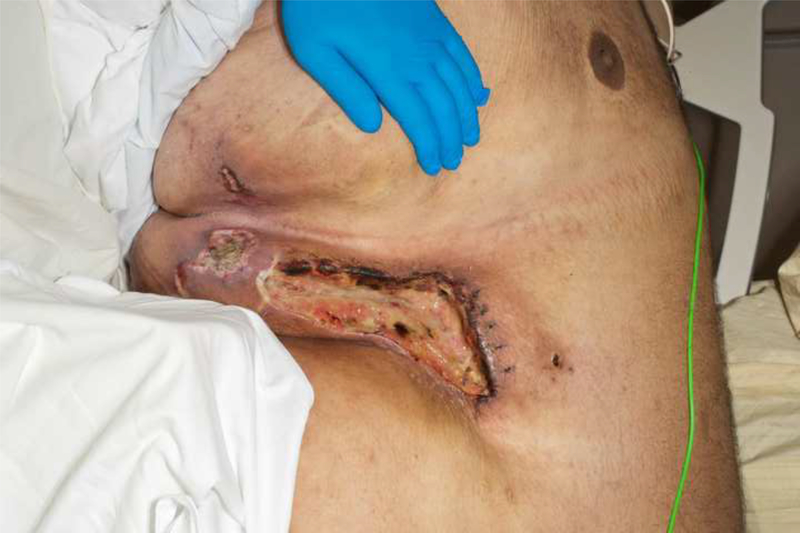
A 28-year-old Caucasian male was evaluated for worsening pain from large skin wounds. His past medical history consisted of super obesity (314kg; 177cm; BMI 100.2kg/m2) and its sequelae—biventricular systolic heart failure; chronic hypoxic and hypercapnic respiratory failure secondary to obstructive sleep apnea and obesity-hypoventilation syndrome managed with nightly continuous positive airway pressure (CPAP); recurrent venous thromboembolism on warfarin; and depression. During the 3 months prior to presentation, the patient fell twice leading to hospitalizations. Deconditioning led to a 71kg weight loss, which likely included both muscle and adipose tissue. The patient developed palpable intertriginous subcutaneous nodules, which subsequently erupted into foul-smelling ulcers that were minimally responsive to prolonged courses of parenteral antibiotics. Palliative care was consulted early during the admission due to exquisitely painful ulcerations in his intertriginous areas and flank (Figure 1).
Figure 1:
Photographs of the patient’s clinical findings including A) Necrotic ulcer with eschar formation and surrounding induration in the right mid-axillary line near the flank; B) close up of anterior chest wall lesion pictured in (A); and C) posterior aspect of right flank.
The patient struggled with obesity since childhood and several of his relatives had obesity. His heaviest weight was 385kg and despite referral for bariatric surgery, he was considered to have a high perioperative risk. The patient had several obesity-related complications including prolonged hospital courses requiring intensive care unit (ICU) care. He had a high school level education and was on disability due to limited mobility and health issues. He lived with his mother who assisted him with toileting, bathing and dressing.
The interdisciplinary team comprised of specialists in wound care, surgery, dermatology, anesthesia, pharmacy, respiratory therapy, palliative medicine, and nursing, provided pain management while undergoing aggressive wound care. A multimodal pain management approach was implemented using duloxetine, pregabalin, acetaminophen, and topical morphine and lidocaine/amitriptyline/ketamine creams, the latter of which were applied to the open wounds. Opioids requirements exceeded 1000 oral morphine equivalents/day. He underwent twice daily bedside dressing changes and surface debridement with conscious sedation consisting of intravenous fentanyl and sublingual ketamine (dosed on ideal body weight), as well as weekly deep surgical debridement. CPAP was used with bedside procedures and postoperatively. Despite multimodal therapy, the patient continued to experience agonizing pain, which limited wound care and resulted in further necrosis and superinfection.
Though initial goals focused on life prolongation and improved pain control, growing concerns about airway protection and reduced respiratory reserve arose, which limited further analgesic titration. Additionally, the patient experienced difficult intubations and hemodynamic instability intraoperatively with debridement.
In line with his goals, the patient transferred to the medical ICU and embarked on a time-limited trial of tracheostomy placement with ventilator support and twice-weekly deep surgical debridement. Baseline analgesia included a continuous fentanyl infusion and patient-controlled boluses for incident pain. Pain with bedside debridement was managed with periprocedural propofol and frequent fentanyl boluses. Previous non-opioid adjuvants were continued. Continuous intravenous ketamine was resumed, this time being dosed on total body weight. Benzodiazepines provided anxiolysis and mitigation of psychotropic effects of ketamine. Nursing used the Richmond Agitation Sedation Score goal of −1 (drowsy) as a target for titrating analgesics. (7) With this improved regimen, the patient’s pain was more optimally controlled using only one-fourth of the total daily opioid dose that he required previously. Regional and neuroaxial techniques were considered but contraindicated due to body habitus, wounds at multiple levels, infection risk, and need for anticoagulation.
Despite aggressive wound care, existing wounds deteriorated and new ones appeared. More intensive wound debridement was required though the multidisciplinary team questioned if this was warranted because the wounds were unlikely curable in this situation. Goals of care were revisited by the interdisciplinary team and the patient requested to be transitioned to a comfort-directed approach after learning there was a dismal chance of the wounds healing. Dressing changes were minimized with a focus on odor control and comfort, and the patient was enrolled in home hospice—using Bi-level Positive Airway Pressure support, parenteral hydromorphone via a battery operated, and sublingual ketamine to facilitate hospital discharge. The patient died comfortably at home five days later.
Comment
We present a case regarding the challenges experienced by the interdisciplinary team in managing pain in a patient with super obesity. The patient’s pain management created a therapeutic dilemma because of unpredictable pharmacokinetics, unreliable weight-based dosing, risk of airway compromise and an existential contribution to suffering. Understanding the impact of body adiposity on pharmacokinetics and pharmacodynamics is necessary to ensure safe, effective pharmacotherapy. Interdisciplinary team input is required.
Several direct, but not readily available, methodologies exist to measure directly an individual’s body composition (e.g. underwater weighing, skinfold measurement, bioelectrical impedance analysis, dual-energy x-ray absorptiometry, computed tomography, or magnetic resonance imaging). As a result, several indirect measures have been developed and used in pharmacology literature and clinical studies (8). See Table 1 for an overview of indirect measurements relying on height, weight, and sex. A major limitation of these indirect anthropometric measures is that they fail to distinguish lean from fat mass. (9, 10) Clinicians need guidance regarding which measures of body mass are most appropriate when calculating drug dosage.
Table 1:
Calculating estimates of body composition. TBW=total body weight (in kilograms unless specified); Height in centimeters, unless specified.
| Indirect Measure | Formula | Comments |
|---|---|---|
| Body mass index (BMI) | TBW ÷ Height 2 | Classification, not dosing scalar Does not distinguish between adipose tissue and lean muscle mass |
| Body surface area (BSA) | TBW0.435 × Height0.725 × 0.007184 | Used to determine dosages of anticancer agents in the non-obese Uncertain use as dosing scalar in obese |
|
Ideal bodyweight (IBW) |
45.4 kg (49.9 kg if male) + 0.89 × (height in cm – 152.4). | Considers sex and height only, not body habitus Sometimes used as dosing scalar |
| Adjusted bodyweight | IBW + 0.4 (TBW-IBW) | Adds to IBW some proportion of difference between TBW and IBW |
| Lean bodyweight (LBW) | A × TBW – B(BMI × TBW) If Male, A=1.1, B=0.0128 If Female, A=1.07, B=0.0148 |
Weight devoid of almost all adipose tissue (extracellular fluid, muscle, bone, vital organs) Sometimes used as a dosing scalar |
Pharmacokinetic Consideration
The three key pharmacokinetic measures that are altered in those with obesity include volume of distribution (Vd), drug clearance (CL) and elimination half-life (t½) (see Table 2) (14) Individuals with obesity have increased splanchnic blood flow, which would be expected to decrease drug bioavailability. (11) However, studies have shown that the bioavailability of drugs with substantial hepatic extraction is not significantly different between obese and lean individuals suggesting that drug absorption and bioavailability do not appear to be altered in obese individuals. (12,13)
Table 2:
Obesity Effect on Pharmacokinetic Properties
| Pharmacokinetic Property | Definition | Obesity Effect on Pharmacokinetic Property |
|---|---|---|
| Absorption and Bioavailability | Fraction of administered dose that reaches systemic circulation | Not altered in oral drugs |
| Volume of distribution (Vd) | Proportionality factor that relates the amount of drug in the body to the plasma concentration Vd is based on physiochemical properties (e.g. lipophilicity, molecular size, degree of ionization, ability to cross biological membranes). Principal parameter determining loading-dose |
Obesity will increase Vd, however weight-adjusted Vd will be smaller than that of normal weight patients for many drugs Highly lipophilic drugs will have markedly increased Vd in obese patients |
| Drug clearance (CL) | Relates the rate of elimination to the plasma concentration Primary parameter determining maintenance dose |
CL increases somewhat proportionally with body weight Obesity may decrease CYP3A4 activity, therefore CL may be decreased for drugs metabolized by CYP3A4 pathway |
| Elimination half-life (t½) | Time required to reduce the plasma concentration of a drug by half | Increases in Vd and/or decreases in CL can prolong t½. Highly lipophilic drugs can accumulate in adipose tissue, leading to risk of side effects and possible toxicity |
Volume of Distribution (Vd)
Vd relates the total amount of a drug in the body to the concentration of the drug in a given compartment. Vd is the principal parameter determining loading-dose selection, and is dependent on a drug’s physiochemical properties impacting the drug’s distribution into tissue. These factors include molecular size, degree of ionization, lipid solubility, and ability to cross biological membranes. (14) Individuals with obesity have an increased absolute and proportional amount of adipose tissue as compared to individuals without obesity. A highly lipophilic drug will have extensive distribution into adipose tissue, which will markedly increase its Vd in patients who are obese. Generally, if a drug is highly lipophilic, the loading dose should be based on total body weight. A hydrophilic drug will not readily distribute into adipose tissue, so its Vd is similar in lean and obese individuals. Therefore, if a drug is hydrophilic, the loading dose should be based on ideal body weight. For this patient, the total and ideal body weights were disparate, with total body weight being 4 times that of his ideal body weight. This initially made many providers uncomfortable with weight-based calculations, which led to subtherapeutic dosing.
Wide variation in the impact of Vd remain, since each drug’s affinity for excess adipose tissue is unique. For example, remifentanil is highly lipophilic but shows minimal to no change in Vd in individuals who are obese. (15) Additionally, plasma protein binding also influences Vd, though obesity does not appear to impact drug’s binding to albumin. Data from studies investigating drug binding to alpha1-acid glycoprotein—a major plasma protein responsible for binding basic drugs and lipoproteins—in obese individuals have been contradictory. (16–18) Tissue blood flow also affects Vd as does cardiac performance, and adipose tissue blood flow may be altered in obesity, potentially altering Vd.
These concepts have relevance to our case, as multiple lipophilic medications were used for analgesia and sedation including propofol, ketamine, fentanyl, methadone, and midazolam. (See Table 3) The desired analgesic and undesired psychotropic effects of ketamine developed for this patient only after its loading dose was increased and adjusted based on total body weight. Pain management during dressing changes improved when his fentanyl boluses were increased four-fold, which actually resulted in less total opioid used.
Table 3.
Suggested dosing guidelines of commonly used palliative care medications in patients with super obesity1
| Medication | Loading Dose | Maintenance Dose |
|---|---|---|
| Highly lipophilic (e.g. ketamine, fentanyl, methadone, diazepam, lorazepam, midazolam, propofol) | Loading dose should be based on total body weight Note - a ceiling dose at the extremes of obesity remains unknown |
Maintenance dose should be based on lean body weight, particularly if used chronically |
| Hydrophilic | Loading dose should be based on ideal body weight | Maintenance dose should be based on lean body weight, particularly if used chronically |
| Drugs metabolized via CYP3A4 (substrates) 2 e.g. fentanyl, oxycodone [partially], alprazolam, midazolam, flurazepam, triazolam, carbamazepine, buspirone) |
Use with caution due to reduced metabolism | |
| Methadone | Consider avoiding due to long elimination half-life resulting in accumulation in adipose tissues, increasing risk of rapid onset respiratory depression | |
| Drugs with a narrow therapeutic index | Use with caution. Dose adjustments should be based on drug plasma concentrations | |
Note these are guidelines, dosing need to be individualized.
Ogu CC, Maxa JL. Drug interactions due to cytochrome P450. BUMC Proceedings 2000; 13:421–423.
As a class, benzodiazepines are relatively lipophilic compounds, particularly midazolam and diazepam. (See Table 4) Therefore, the loading dose of these medications should be based on total body weight. (19) However, a ceiling dose at the extremes of obesity remains unknown. In our patient, midazolam loading dosing was doubled, so as not to result in excessive sedation. Once again, the current evidence indicates that changes with Vd observed in patients with obesity are drug-specific, but must be accounted for when considering dosage in this population.
Table 4:
Opioid and Benzodiazepine Lipophilicity based on octanol: water partition coefficient
| Opioids |
Benzodiazepines |
|
|---|---|---|
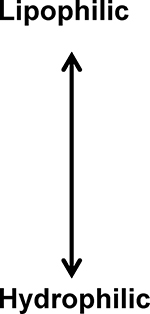 |
Sufentanil Fentanyl Alfentanil Methadone Meperidine Remifentanil Hydromorphone Morphine Oxycodone |
Midazolam Diazepam Chlordiazepoxide Lorazepam Oxazepam Alprazolam |
Drug Clearance (CL)
CL is defined as the volume of blood from which the drug is completely removed during a given time. Put differently, CL relates the rate of elimination to the plasma concentration. CL depends on blood flow to an organ and that organ’s ability to extract the drug from the blood. Unlike Vd, CL is largely controlled by hepatic and renal physiology, which may be altered in obesity. CL is inversely related to the steady-state plasma concentration and is the primary determinant when calculating maintenance dosage.
The liver is primarily responsible for drug metabolism and plays an integral role in mediating CL. Nonalcoholic fatty liver disease in individuals with obesity may alter hepatic blood flow, and may have an impact on CL. (20) Increases in CYP2D6 have been observed in individuals with obesity. Also, the activity of CYP3A4—a major enzyme responsible for the metabolism of opioids—appears not to be increased proportionally with increased body weight; rather, there is variability among individual patients. Thus, it appears likely that patients with super obesity may have less capacity to utilize this metabolic pathway. (20) CL may be altered for drugs metabolized via these pathways for patients with obesity. (14) Therefore, drugs metabolized via CYP3A4 (e.g. fentanyl, alprazolam, midazolam, oxycodone [partially]) should be used with caution given the reduced metabolism exhibited by patients with extreme obesity.
The kidney is the other primary organ involved in CL; however, the effects of obesity on glomerular filtration rate (and thus creatinine clearance), renal tubular secretion, and tubular reabsorption remain unclear. Overall studies demonstrated three major observations regarding creatinine clearance in the obese. (21) First, a higher absolute renal CL is observed in obesity compared to normal weight counterparts. Second, renal CL does not increase linearly with total body weight. Lastly, some studies suggest renal CL and lean body weight correlate linearly. It is recommended that clinicians base maintenance doses of on lean body weight (or a similar descriptor) that corrects for differences in absolute CL between obese and non-obese individuals, particularly if the drug is used chronically. The validity of this, however, remains untested at the extremes of obesity. For our patient, maintenance doses were based on lean body weight.
Elimination half-life (t½)
The elimination half-life (t½) of a drug depends on both the Vd and CL. Increases in Vd and/or decreases in CL can prolong t½. In our patient, medications with increased Vd, such as midazolam, had prolonged elimination t½. If this medication were used chronically, it could be burdensome due to its gradual accumulation in fatty tissue and increased the risk of adverse events. Medications like fentanyl, propofol and methadone would also be expected to have an extended elimination t½. Subsequent doses of lipophilic drugs should be dosed cautiously. It will typically appear that the patient is tolerating the first few doses very well, leading the clinician to believe that the higher dosing strategy should be continued. However, as the adipose tissue becomes saturated with drug, accumulation occurs and can lead increasing the risk of side effects and possible toxicity, such as rapid onset of respiratory depression. As this is particularly true for methadone, which has biphasic elimination (a shorter t½ and a longer t½) based on its lipophilicity, we chose to avoid methadone for this patient.
Unfortunately, the literature is very limited in this regard, as study participants with obesity are often excluded from clinical trials during drug development. Furthermore, trials conducted in such populations are often drug-specific, may exclude the super obese subgroup, and may use varying measures of body composition.
Final Considerations
Structural issues from super obesity itself can make respiratory failure a serious concern due to underlying hypoventilation and risk of airway obstruction in the setting of obstructive sleep apnea. For our patient, this prompted use of both non-invasive and invasive ventilation to mitigate any limitations that respiratory status might have posed in optimally controlling pain.
Analgesia was especially challenging due to unpredictable pharmacokinetics, inability to use weight-based dosing reliably, and risk of airway compromise. These factors alone do not even account for the significant suffering the patient experienced from social isolation, guilt regarding his medical conditions, and existential concerns—which added complexity to our case.
Our patient presented with significant weight loss prior to presentation from deconditioning, resulting in loss of muscle mass and function, frequently termed sarcopenia. Sarcopenic obesity predicts worse clinical outcomes such as physical disability, increased infection risk and reduced survival when comparing those with sarcopenic obesity to obesity alone (22, 23) Nevertheless, there is limited data on changes in pharmacokinetics and pharmacodynamics in the populations with sarcopenic obesity and this observation is an extrapolation from extant literature.
Overall, pharmacokinetic data in patients with super obesity is sparse for most drugs. To that end, it appears prudent that drugs with a narrow therapeutic index (range of efficacy between subtherapeutic and toxic levels) should be used cautiously and dose adjustments should be based on drug plasma concentrations, if available. Close patient monitoring is essential to titrate drugs optimally to desired clinical effect. As the prevalence of obesity increases, clinicians need to be aware of these pathophysiologic changes to provide optimal symptom management.
Acknowledgements
Abstract was presented at the 2015 Annual Assembly of the American Academy of Hospice and Palliative Medicine (AAHPM) and the Hospice and Palliative Nurses Association (HPNA), Philadelphia, Pennsylvania, February 26, 2015.
Dr. Batsis is supported in part by the National Institute On Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Dartmouth Health Promotion and Disease Prevention Research Center (Cooperative Agreement Number U48DP005018) from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturm R Increases in morbid obesity in the USA: 2000–2005. Public Health 2007; 121:492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters A, Barendregt JJ, Willekens F et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2013; 138:24–32. [DOI] [PubMed] [Google Scholar]
- 4.Andreyeva T, Strum R, Ringel JS. Moderate and severe obesity have large differences in health care costs. Obes Res 2004; 12:1936–43. [DOI] [PubMed] [Google Scholar]
- 5.Cheymol G Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000; 39:215–31. [DOI] [PubMed] [Google Scholar]
- 6.Renquist K Obesity classification. Obes Surg 1998; 8:480. [DOI] [PubMed] [Google Scholar]
- 7.Sessler CN, Gosnell MS, Grap MJ et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 1668:1338–44. [DOI] [PubMed] [Google Scholar]
- 8.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 2004; 58:119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okorodudu DO, Jumean MF, Montori VM et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes 2010; 34:791–9. [DOI] [PubMed] [Google Scholar]
- 10.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes 2016; 40:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull 1962;1:39–44. [PubMed] [Google Scholar]
- 12.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity in midazolam kinetics. Anesthesiology 1984; 61:27–35. [PubMed] [Google Scholar]
- 13.Bowman SL, Hudson SA, Simpson G, Munro JF, Clements JA. A comparison of the pharmacokinetics of propranolol in obese and normal volunteers. Br J Clin Pharmacol 1986; 24:529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of Obesity on the Pharmacokinetics of Drugs in Humans. Clin Pharmacokinet 2010:49(2) 71–87. [DOI] [PubMed] [Google Scholar]
- 15.Egan TD, Huizinga B, Gupta SK, et al. Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology. 1998; 89: [DOI] [PubMed] [Google Scholar]
- 16.Cheymol G, Poirier JM, Barre J, Pradalier A, Dry J. Comparative pharmacokinetics of intravenous propranolol in obese and normal volunteers. J Clin Pharmacol 1987; 27:874–9. [DOI] [PubMed] [Google Scholar]
- 17.Derry CL, Kroboth PD, Pittenger AL, Kroboth FJ, Corey SE, Smith RB. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol 1995; 15:197–205. [DOI] [PubMed] [Google Scholar]
- 18.Benedek IH, Blouin RA, McNamara PJ. Serum protein binding and the role of increased alpha 1-acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol 1984; 18:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenblatt DJ, Arendt RM, Abernethy DR, Giles HG, Sellers EM, Shader RI. In vitro quantitation of benzodiazepine lipophilicity: relation to in vivo distribution. Br J Anaesth. 1983; 55:985–9. [DOI] [PubMed] [Google Scholar]
- 20.Ghobadi C, Johnson TN, Aarabi M, et al. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin Pharmacokinet. 2011; 50:809–22. [DOI] [PubMed] [Google Scholar]
- 21.Han PY, Duffull SB, Kirkpatrick CM, Green B. Dosing in Obesity: A Simple Solution to a Big Problem. Clin Pharmacol Ther 2007; 82 (5): 505–8. [DOI] [PubMed] [Google Scholar]
- 22.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012; 31:583–601. [DOI] [PubMed] [Google Scholar]
- 23.Batsis JA, Mackenzie TA, Emeny RT, Lopen-Jimenez F, Bartels SJ. Low Lean Mass With and Without Obesity, and Mortality: Results From the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2017. February 16. doi: 10.1093/gerona/glx002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]