Table 2.
Overview of the bond energies (BE), reaction enthalpies (ΔHr), proton affinities (PA), and protonated structures, calculated at the CCSD(T)-F12/VDZ-F12//ωB97X-D/aug-cc-pVTZ level of theory at 298 K.
| Compound (A) | BE [kcal/mol] | ΔHr [kcal/mol] | PAcalc [kcal/mol] | PAliterature [kcal/mol] | Protonated structure |
|---|---|---|---|---|---|
| Ammonia | 203.8 | 204a | |||
| Acetone | 26.4 | 35.7 | 194.5 | 194a | 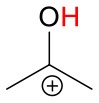 |
| Methyl vinyl ketone (MVK) | 27.3 | 32.9 | 198.2 | 199.5a |  |
| Methyl ethyl ketone (MEK) | 25.9 | 34.0 | 195.7 | 197.7a | 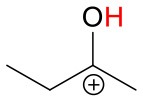 |
| α-Pinene | 17.9 | 15.4 | 206.3 | 204–209b, c | 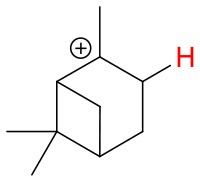 |
| β-Pinene | 18.2 | 13.4 | 208.7 | 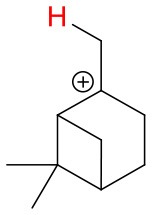 |
|
| Camphene | 18.5 | 15.2 | 207.2 | 205.7c | 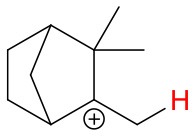 |
| 3-Carene | 20.6 | 19.0 | 205.4 |  |
|
| 27.8 | 196.7 | 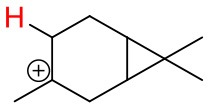 |
|||
| Limonene | 22.3 | 25.0 | 201.2 | 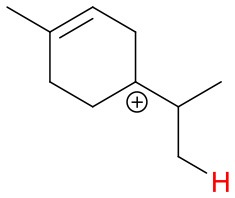 |
|
| 26.0 | 200.2 | 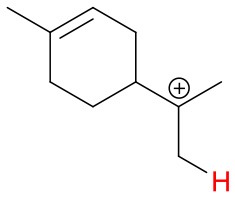 |
|||
| Myrcene | 20.9 | 20.5 | 204.2 | 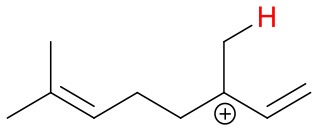 |
|
| Ocimene | 26.0 | 19.1 | 210.7 | 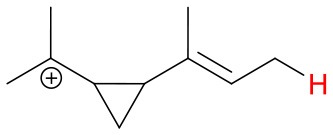 |
|
| Sabinene | 20.6 | 8.5 | 215.9 | 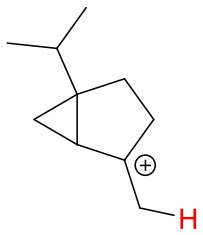 |
BE describes the -A bond energy, ΔHr is the reaction enthalpy of the reaction . We expect errors in computed proton affinities and binding enthalpies to be smaller than 1 kcal/mol. PA literature values are also given if available.
(Hunter and Lias, 1998).
(Lindinger et al., 1998b).
(Solouki and Szulejko, 2007).
