Figure 1.
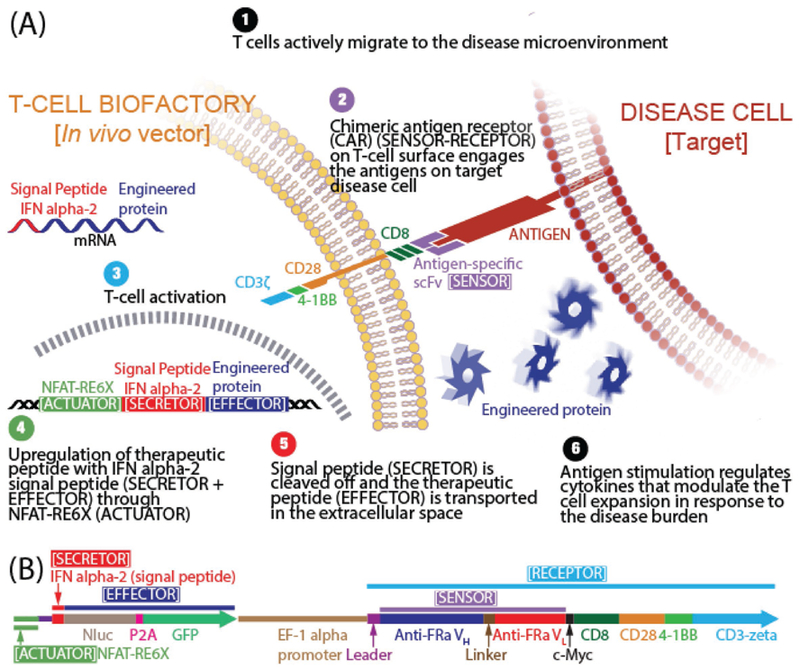
Schematics. A) T-cell Biofactory. B) Artificial cell-signaling pathway comprises of three constant domains (Receptor, Actuator, Secretor) and two variable domains (Sensor, Effector) arranged in cis. [(i) Receptor: Based on the chimeric antigen receptor (CAR) (GenBank: HJ220879.1) from clinical CAR T-cell trials (ref. [11]), containing the CD3-zeta signaling domain of the TCR-CD3 complex that causes intracellular calcium ([Ca2+]i) rise upon engaging its target antigen (ref. [12]). Briefly, the extracellular portion of the CAR is composed of the CD8 segment proximal to the cell membrane. The transmembrane portion that connects the extracellular and intracellular domains is from CD28 and extends as the intracellular costimulatory domain. Additional 4–1BB costimulatory domain found to enhance the therapeutic response of CAR T cells by mitigating early exhaustion of T cells engineered to express antigen-specific CARs was included. (ii) Actuator: Based on six copies of NFAT-RE (ggagga aaaact gtttca tacaga aggcgt) that respond to the [Ca2+]i rise (ref. [10]) and placed upstream of a transcriptional start site for the Effector transgene. (iii) Secretor: Derived from human interferon alpha-2 (IFNa2) (ref. [13]) (GenBank: NM_000605). (iv) Sensor (part of the transmembrane Receptor): Comprises of variable domains from heavy (VH) and light (VL) chain of the antigen-specific antibody. In this work, these were derived from the anti-FRa antibody (ref. [7]) (MORAb-003) or the anti-MSLN antibody (ref. [8]) (MORAb-009). (v) Effector: Comprises of a reporter transgene that can be exchanged with a therapeutic transgene to neutralize the pathology that triggered the T-cell Biofactory. In this work, Nanoluc luciferase reporter was used (Promega, GenBank: JQ437370.1).]
