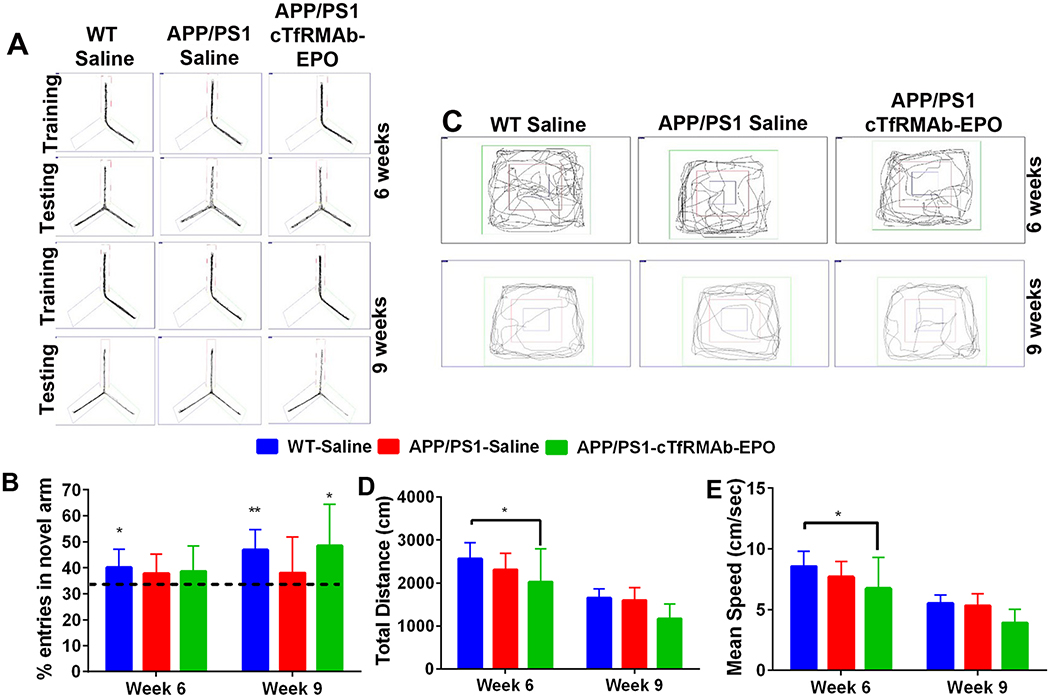
Figure 7. Effect of the cTfRMAb-EPO fusion protein on spatial memory and exploration in the APP/PS1 mice.
Representative trajectories of saline treated WT, saline- and cTfRMAb-EPO-treated APP/PS1 mice during the training and testing phase of the Y-maze (A). Six-weeks after treatment initiation, saline treated WT mice showed a preference for the novel arm however, nine-weeks after treatment initiation, both the saline treated WT and cTfRMAb-EPO treated APP/PS1 mice showed a preference for the novel arm (B). Representative trajectories of saline treated WT, saline- and cTfRMAb-EPO-treated APP/PS1 mice during the open-field test (C). Six-weeks after treatment initiation, cTfRMAb-EPO treated APP/PS1 mice had significantly lower distance travelled (D) and mean speed (E) compared with saline treated WT mice. At 9 weeks, no differences in distance travelled or mean speed were observed between any experimental groups (D-E). Data are presented as mean ± SD of 8–10 mice per group. For Y-maze, one-sample t test with a hypothesized value of 33% (indicated by the dotted line). Two-way repeated measures ANOVA for the open-field analysis with Bonferroni’s correction. *p<0.05, **p<0.01.