Abstract
Background
People with asthma have a higher prevalence of anxiety and depression than the general population. This is associated with poorer asthma control, medication adherence, and health outcomes. Cognitive behavioural therapy (CBT) may be a way to improve the quality of life of people with asthma by addressing associated psychological issues, which may lead to a lower risk of exacerbations and better asthma control.
Objectives
To assess the efficacy of CBT for asthma compared with usual care.
Search methods
We searched the Cochrane Airways Group Specialised Register, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). We also searched reference lists of all primary studies and review articles and contacted authors for unpublished data. The most recent searches were conducted in August 2016.
Selection criteria
We included parallel randomised controlled trials (RCTs) comparing any cognitive behavioural intervention to usual care or no intervention. We included studies of adults or adolescents with asthma, with or without comorbid anxiety or depression. We included studies reported as full text, those published as abstract only, and unpublished data.
Data collection and analysis
Two or more review authors independently screened the search results, extracted data, and assessed included studies for risk of bias. We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMD) where scales varied across studies, all using a random‐effects model. The primary outcomes were asthma‐related quality of life and exacerbations requiring at least a course of oral steroids. We rated all outcomes using GRADE and presented our confidence in the results in a 'Summary of findings' table.
Main results
We included nine RCTs involving 407 adults with asthma in this review; no studies included adolescents under 18. Study size ranged from 10 to 94 (median 40), and mean age ranged from 39 to 53. Study populations generally had persistent asthma, but severity and diagnostic measures varied. Three studies recruited participants with psychological symptomatology, although with different criteria. Interventions ranged from 4 to 15 sessions, and primary measurements were taken at a mean of 3 months (range 1.2 to 12 months).
Participants given CBT had improved scores on the Asthma Quality of Life Questionnaire (AQLQ) (MD 0.55, 95% confidence interval (CI) 0.17 to 0.93; participants = 214; studies = 6; I2 = 53%) and on measures of asthma control (SMD ‐0.98, 95% CI ‐1.76 to ‐0.20; participants = 95; studies = 3; I2 = 68%) compared to people getting usual care. The AQLQ effect appeared to be sustained up to a year after treatment, but due to its low quality this evidence must be interpreted with caution. As asthma exacerbations requiring at least a course of oral steroids were not consistently reported, we could not perform a meta‐analysis.
Anxiety scores were difficult to pool but showed a benefit of CBT compared with usual care (SMD ‐0.38, 95% CI ‐0.73 to ‐0.03), although this depended on the analysis used. The confidence intervals for the effect on depression scales included no difference between CBT and usual care when measured as change from baseline (SMD ‐0.33, 95% CI ‐0.70 to 0.05) or endpoint scores (SMD ‐0.41, 95% CI ‐0.87 to 0.05); the same was true for medication adherence (MD ‐1.40, 95% CI ‐2.94 to 0.14; participants = 23; studies = 1; I2 = 0%).
Subgroup analyses conducted on the AQLQ outcome did not suggest a clear difference between individual and group CBT, baseline psychological status, or CBT model. The small number of studies and the variation between their designs, populations, and other intervention characteristics limited the conclusions that could be drawn about these possibly moderating factors.
The inability to blind participants and investigators to group allocation introduced significant potential bias, and overall we had low confidence in the evidence.
Authors' conclusions
For adults with persistent asthma, CBT may improve quality of life, asthma control, and anxiety levels compared with usual care. Risks of bias, imprecision of effects, and inconsistency between results reduced our confidence in the results to low, and evidence was lacking regarding the effect of CBT on asthma exacerbations, unscheduled contacts, depression, and medication adherence. There was much variation between studies in how CBT was delivered and what constituted usual care, meaning the most optimal method of CBT delivery, format, and target population requires further investigation. There is currently no evidence for the use of CBT in adolescents with asthma.
Plain language summary
Cognitive behavioural therapy for people with asthma
Take‐home message
Cognitive behavioural therapy (CBT) may improve the quality of life and asthma control of adults with asthma, but there is limited evidence for other important outcomes, and our confidence in the results is quite low. None of the studies included adolescents with asthma.
Review question
We wanted to review the evidence of the effect of CBT compared to usual care (without CBT) on a range of health outcomes in people with asthma including quality of life, medication adherence, and levels of anxiety and depression.
Background
People with asthma suffer from anxiety and depression more than the general public. These psychological problems are linked with having worse asthma, including having poorer control of symptoms and being admitted to hospital more often. CBT is a talking therapy that aims to help people recognise how their behaviour affects their thoughts and feelings, which may help people with asthma better cope with their condition. We wanted to learn whether using CBT was better than not using CBT for improving the lives of people with asthma.
Study characteristics
The evidence reviewed is current to August 2016. We included nine studies with a total of 407 participants in the review. All of the participants had asthma. In three of the nine studies, the participants also had a diagnosis of anxiety or depression, or both. The CBT was given either individually or in a group and ranged from four to 15 sessions.
Key results
Participants given CBT had improved scores on the Asthma Quality of Life Questionnaire (AQLQ) and on measures of asthma control compared to participants who did not receive CBT. The studies generally did not report whether CBT reduced the likelihood of people needing oral steroids for an asthma attack. The benefit on AQLQ score was sustained up to a year after receiving CBT. Participants given CBT also had better anxiety scores compared to those given usual care. Participants given CBT did not have clearly improved depression scale scores or medication adherence.
The overall quality of evidence presented is low due to the small number of studies included in the review, the differences in the design of the studies and in how the CBT was conducted, and because the participants knew to which treatment group (CBT or no CBT) they had been assigned.
Summary of findings
Summary of findings for the main comparison. Cognitive behavioural therapy versus usual care.
| Cognitive behavioural therapy (CBT) for adults and adolescents with asthma | ||||||
|
Patient or population: adults and adolescents with asthma
Setting: outpatient care
Intervention: CBT
Comparison: usual care (some variation in control group definitions among studies such as "no treatment", "waiting list") The weighted mean outcome assessment was taken at 3.3 months (range 1.2 to 12 months). | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care** | Risk with CBT | |||||
|
Asthma‐related quality of life (AQLQ)
1 to 7 scale (higher scores better) |
The mean change in AQLQ score in the usual care group was 0.53. | The mean AQLQ score in the intervention group was 0.55 better (0.17 better to 0.93 better). | ‐ | 214 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Benefit of CBT over usual care The MCID on the AQLQ is 0.5 units. |
| Asthma exacerbations requiring at least a course of oral steroids | Analysis not possible due to inconsistent definitions, baseline imbalances, and incomplete diary data. | ‐ | ‐ | Not graded | Results are reported narratively in the review. | |
|
Asthma control Mean change on the ASC and ACQ (adjusted so lower scores are better) |
It was not possible to derive a meaningful control group risk because different scales were used. | The mean asthma control in the intervention group was 0.98 standard deviations better (1.76 better to 0.2 better). | ‐ | 95 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 3 4 | Benefit of CBT over usual care, but significant variation in results. |
|
Unscheduled healthcare visits Mean visits per participant in the 6 months after treatment (lower scores better) Primary care visits included nurse and out‐of‐hours visits |
The usual care group had a mean 2.08 GP visits. | There were 0.28 fewer unscheduled GP visits in the intervention group (1.36 fewer to 0.8 more). | ‐ | 80 (1 RCT) | ⊕⊕⊝⊝ LOW 5 6 | No evidence of a benefit of CBT over usual care. |
| The usual care group had a mean 2.27 primary care visits. | There were 0.40 fewer unscheduled primary care visits in the intervention group (1.51 fewer to 0.71 more). | |||||
|
Anxiety scales Mean change on the ASC panic/fear, PSS, and HADS‐Anxiety (lower scores better) |
It was not possible to derive a meaningful control group risk because different scales were used. | The mean change in the intervention group was 0.38 standard deviations better (0.73 better to 0.03 better) | ‐ | 225 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 7 8 9 | Possible small benefit of CBT over usual care Our confidence was reduced by a smaller and less precise result from 3 more studies (n = 142) reporting endpoint scores (SMD ‐0.25, 95% CI ‐1.02 to 0.51). |
|
Depression scales
Mean change on HADS‐Depression.
Endpoint scores on NEM, BDI, and QD (see comment) (lower scores better) |
The usual care group showed a mean change on the HADS‐Depression of ‐1.7 units. | The mean change in the intervention group was 0.33 standard deviations better (0.70 better to 0.05 worse). | ‐ | 112 (2 RCTs) |
⊕⊕⊝⊝ LOW 1 9 10 11 | Possible small benefit of CBT over usual care, but confidence intervals include no difference. 3 more studies (n = 83) reporting endpoint scores on various scales showed a similar result (SMD ‐0.41, 95% CI ‐0.87 to 0.05). |
| Medication adherence 6‐item Adherence Scale rated 1 to 5 (lower scores better) | The mean medication adherence in the usual care group was 8.4. | The mean medication adherence in the intervention group was 1.4 units better (2.94 better to 0.14 worse). | ‐ | 23 (1 RCT) | ⊕⊕⊝⊝ LOW 12 | Possible small benefit of CBT over usual care, but confidence intervals include no difference. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **The risk in the control group is based on the usual care scores in each study contributing to the analysis. For continuous outcomes, this could not include studies reporting mean difference between groups. ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ASC: Asthma Symptom Checklist; BDI: Beck Depression Inventory; CI: confidence interval; GP: general practitioner (family doctor); HADS: Hospital Anxiety and Depression Scale; MCID: minimal clinically important difference; MD: mean difference; NEM: Negative Emotionality Scale; PSS: Perceived Stress Scale; QD: Depression Questionnaire (in Italian); RCT: randomised controlled trial; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1These subjective rating scales may have been biased by the inability to blind participants and personnel to group assignment. Additionally, some studies contributing to the effect were at high risk of bias due to high or unbalanced dropout (‐1 risk of bias). 2There was important variation between the study results (I2 = 53%, P = 0.06) (‐1 inconsistency). 3There was important variation between the study results (I2 = 68%, P = 0.04) (‐1 inconsistency). 4The effect is based on small numbers of studies and participants randomised, but we did not consider this a sufficient reason to downgrade (no downgrade for imprecision). 5The study was at high risk of performance bias, but it is unclear whether this would have affected the behaviour of participants for this outcome, or the way it was recorded by study personnel. The study was also rated high risk for attrition bias, but we did not consider this sufficient to warrant a downgrade (no downgrade for risk of bias). 6Only one study with 80 participants reported the outcome, and the confidence intervals for the effect made it difficult to tell whether CBT is likely to have any benefit over usual care (‐2 imprecision). 7Statistical heterogeneity in the change scores was not significant (I2 = 28%, P = 0.25), but there was much variation between the endpoint scores shown in the comments for this outcome (I2 = 76%, P = 0.01) and inconsistency between the two analyses (‐1 inconsistency). 8The effect based on change scores was relatively precise, but the endpoint scores analysis was not (no downgrade for imprecision). 9Deshmukh 2008 measured the HADS‐Anxiety and HADS‐Depression, the results of which were not available in the abstract or poster, but the number of participants (n = 12) means the results are unlikely to have been affected (no downgrade for publication bias). 10The confidence intervals did not exclude no difference so it is difficult to tell whether CBT has an important effect on depression (‐1 imprecision). 11Statistical heterogeneity was very high in the analysis of endpoint scores shown in the comments for this outcome (I2 = 80%, P = 0.007), but there was no important variation in the change scores analysis (I2 = 0%, P = 0.58) or between the two depression analyses (no downgrade for inconsistency). 12Only one study with 23 participants reported the outcome, and the confidence intervals for the effect did not exclude no difference between CBT and usual care (‐2 imprecision).
Background
Description of the condition
Asthma is a chronic disease of the airways that causes reversible breathing difficulties due to narrowing of the airways, thickening of the airway walls, and increased mucus production (GINA 2016). These physical characteristics commonly lead to symptoms including wheezing, shortness of breath, chest tightness, and cough, which vary significantly over time and between people (GINA 2016).
Recent estimates suggest that over 334 million people have asthma worldwide, which leads to direct treatment costs and indirect costs to society that are amongst the highest for non‐communicable diseases (Global Asthma Network 2014). The disease is a significant cause of avoidable morbidity and mortality in high‐income countries such as the UK and Australia for patients, their families, and in terms of lost working days (GINA 2016; Global Asthma Network 2014; Royal College of Physicians 2014), and even more so in low‐ and middle‐income countries, where it often goes undiagnosed and untreated (Global Asthma Network 2014).
People with asthma have a higher prevalence of anxiety and depression than the general population (GINA 2016; Zielinski 2000). Depending on the severity of asthma, prevalence of depression has been estimated at between 22% and 45%, and anxiety and panic disorder between 6.5% and 26% (Ettinger 2004; Heaney 2005; Katon 2004; Lavoie 2010; Mancuso 2000). Asthma symptoms can worsen quickly during exacerbations and are often frightening, especially for young people (BTS/SIGN 2014). This can lead to health‐related anxiety and hypervigilance, which can act as a future trigger for asthma (Thoren 2000). Whether asthma causes anxiety and depression, or the psychological disorder precedes an asthma diagnosis, the two can influence each other and make both conditions more difficult to live with (Asthma UK 2015). Adolescents with asthma in particular are at a greater risk of major depression, panic attacks, and anxiety disorders, which have been associated with an increased burden of asthma symptoms and inability to cope with the disease (Richardson 2006). The presence of psychological disorders in people with asthma of any age is associated with poorer asthma outcomes and increased hospital utilisation (GINA 2016), particularly for those from disadvantaged socio‐economic and ethnic backgrounds (Royal College of Physicians 2014).
In asthma, the increased incidence of anxiety and panic disorders in particular is complicated by their overlap in symptoms (Carr 1998; Shavitt 1992), which can mean symptoms of anxiety are often misinterpreted by patients and clinicians (Avner 1988). Symptoms that are common to both conditions include breathlessness, chest tightness, psychogenic cough, palpitations, and inability to complete sentences (Asthma UK 2015; BTS/SIGN 2014). This overlap, and general feelings of not being able to cope, can lead to overuse of bronchodilators, which are associated with serious side effects (FDA 2010). Conversely, depression in asthma can lead to poor adherence with preventative medications and non‐adherence to lifestyle advice (e.g. smoking cessation, recreational drug use, and allergen avoidance), which may increase the likelihood of exacerbations and loss of asthma control (Royal College of Physicians 2014).
Description of the intervention
Cognitive behavioural therapy (CBT) is a form of talking therapy that explores a person’s perceptions of themselves and others and how a person’s behaviour influences their thoughts and feelings. CBT aims to positively change how a person thinks (‘cognitive’) and what they do (‘behaviour’). CBT entails psychological analysis of a specific problem or situation. The specific thoughts, emotions, physical feelings, and actions that relate to this specific problem are explored. A more positive way of thinking about the specific situation or problem is developed and a more helpful behavioural response is aimed for. There are different models and methods of delivering CBT. The classic model of CBT (or so‐called second‐wave CBT) has a strong focus on addressing simple information processing. It has traditionally been delivered face to face either individually or in a group. Online models, which are cheaper to deliver and more accessible for patients, also exist but may be less effective than face‐to‐face therapy (Mayo‐Wilson 2013). Newer ‘third‐wave CBT’ includes a more heterogeneous group of treatments including mindfulness, dialectical‐based therapy, behavioural activation, and schema therapy, among others.
Cognitive behavioural therapy has a large evidence base and is effective for a range of psychological disorders, which has resulted in it being recommended in a range of treatment guidelines (e.g. depression, generalised anxiety disorder, social anxiety) (NICE 2009; NICE 2011; NICE 2013). Most research into CBT focuses on people with mental health problems, but evidence is growing to support its use in chronic illness, especially as part of self management plans, to help people cope with the psychological aspects of physical illness. These include worrying and painful symptoms, demanding and debilitating treatments and their side effects, fatigue, and lifestyle change (White 2001). CBT has been used in this way for asthma as a way of encouraging patients to accept their problems, keep control of their symptoms and medications, and alleviate anxiety related to their condition (Grover 2002; Kotses 1995).
Therapies vary in the specific components used and in the delivery and duration of treatment. They are usually based on a structured manual that can be adapted according to the individual's particular problems, and can be delivered for between 5 and 20 weekly or fortnightly sessions of 30 to 60 minutes (Royal College of Psychiatrists 2015).
How the intervention might work
Cognitive behavioural therapy is "a way of talking about how you think about yourself, the world and other people [and] how what you do affects your thoughts and feelings" (Royal College of Psychiatrists 2015). In the context of chronic diseases, a person might find certain aspects of their disease worrying or difficult to deal with. In some situations this might be realistic, but the extent of worry, panic, or sadness may be exaggerated compared with the actual threat, and may cause them to behave differently (avoiding certain activities, taking too much medication). This in turn leads to physiological responses that are misinterpreted to reinforce and maintain their unhelpful behaviours and fears (Figure 1). CBT aims to break this cycle by encouraging people to challenge their unhelpful thoughts and form more realistic ones based on what is more likely to happen, and confront situations or activities that worry them.
1.
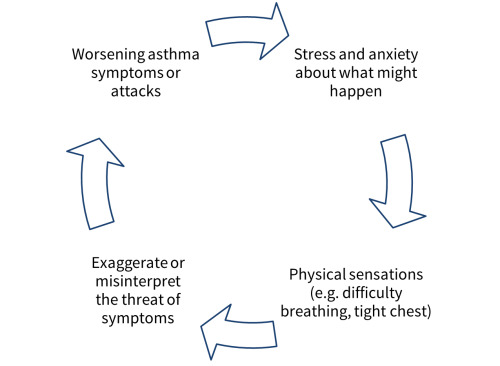
Cycle of worry in asthma.
Why it is important to do this review
The psychological aspects of asthma are associated with increased morbidity and mortality, which may be partially explained by an association between depression and anxiety and poor adherence with medicines (DiMatteo 2000). In asthma, poor psychological well‐being has been associated with an increased burden of asthma symptoms and poor self management, which places greater pressure on health services (GINA 2016; Richardson 2006). It is important to assess the effect of CBT on quality of life to determine whether the treatment can help people to better cope with these psychological and asthma‐related difficulties. We also examined whether CBT has the potential to improve clinical asthma symptoms, particularly the likelihood of needing oral steroids to treat exacerbations, which may result from encouraging better self management and treatment adherence, and improving psychological well‐being.
A Cochrane systematic review of all psychological therapies for asthma published in 2006 was unable to draw any definitive conclusions regarding the effectiveness of these treatments due to variation in the interventions, small trials, and inadequate reporting (Yorke 2006). This review summarised the updated evidence base, focusing on the usefulness of the most widely used and studied psychological intervention, CBT, on an updated set of outcomes.
Objectives
To assess the efficacy of CBT for asthma compared with usual care.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs) of any duration. We excluded trials with a cross‐over design because it is unlikely that the effects of the intervention could be effectively 'washed out' between treatment periods. Due to the nature of the interventions, we anticipated that the studies would be unblinded for participants and personnel, but we included studies irrespective of whether they blinded outcome assessors. We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included studies of adults and adolescents from 12 years of age with a diagnosis of asthma according to internationally recognised guidelines, for example GINA 2016. Participants did not have to have a clinical diagnosis of anxiety or depression to be included. If studies included younger children, we included the study if the mean age of the study population was above 12. We excluded studies of mixed populations (i.e. those recruiting participants with chronic obstructive pulmonary disease (COPD) or other chronic conditions) unless results for people with asthma were presented separately.
Types of interventions
We included studies comparing individual or group CBT with usual care or minimal‐intervention control groups. Relevant therapies included both cognitive and behavioural elements which had a specific focus on tackling negative thoughts and behaviours relating to asthma. We included any model of CBT including acceptance and mindfulness‐based therapies. We included studies that allowed any asthma medications or co‐interventions as long as they were the same for both groups. We included control groups on a waiting list as long as they continued to receive usual asthma care, and minimal‐intervention control groups such as the use of printed materials.
Types of outcome measures
Primary outcomes
Asthma‐related quality of life (measured on a validated scale, e.g. Asthma Quality of Life Questionnaire (AQLQ))
-
Asthma exacerbations requiring at least a course of oral steroids
Due to the variation in reporting of asthma exacerbations, we also considered data for other types of unscheduled healthcare utilisation depending on what was available.
Quality of life is an important outcome that can reflect to what degree asthma affects people's lives. Cognitive behavioural therapy may result in better symptom control by improving adherence and reducing the negative effects of anxiety and depression, but may also help people to accept and deal with symptoms better when they do arise. Looking at asthma exacerbations allowed us to assess whether any positive effect of CBT leads to important clinical benefits.
Secondary outcomes
Asthma control (measured on a validated scale, e.g. Asthma Control Questionnaire (ACQ))
Unscheduled contacts with health services for asthma (i.e. emergency general practitioner appointment, emergency department visit, or hospitalisation)
Validated scales of anxiety
Validated scales of depression
Medication adherence
We did not anticipate 'adverse events' being defined or recorded as they would be in drug studies, but rather as negative events relating to asthma which will fall within 'asthma exacerbations requiring at least a course of oral steroids' or 'unscheduled contacts with health services for asthma'. In this sense, the direction of the effect indicated benefit or potential harm of CBT compared with the control group. If other adverse events were reported that did not fall under these categories, we described them narratively.
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
If different scales measuring the same outcome were used across studies, we pooled them in the same analysis using standardised mean differences if we judged this to be appropriate.
The main time point for measurement was after the CBT intervention had been completed, and we looked at information for long‐term follow‐up separately if it was available.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Group Specialised Register, which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of multiple bibliographic databases and handsearching of respiratory journals and meeting abstracts (Appendix 1). We searched all records in the Cochrane Airways Group Specialised Register using the search strategy illustrated in Appendix 2.
We also conducted a search of ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/). We searched all databases from their inception to August 2016 with no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted authors of included studies regarding ongoing or unpublished trials.
We searched for errata or retractions from included studies published in full on PubMed on 29 January 2016.
Data collection and analysis
Selection of studies
Two review authors (KK and MN or VD) independently screened titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications, and two review authors (KK and MN or VD) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, by consulting a third review author (MN or VD, whoever had not already screened the record). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data that was piloted on at least one study in the review. Two review authors (KK and MN or VD) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (KK and MN or VD) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (either MD or VD, whoever had not already extracted data). One review author (KK) transferred data into Cochrane statistical software (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (KK and MN or VD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (either MD or VD, whoever had not already extracted data).
We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol, Kew 2015, and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios and continuous data as mean differences or standardised mean differences. We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We described skewed data reported as medians and interquartile ranges narratively.
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
For dichotomous outcomes, we used participants rather than events as the unit of analysis (i.e. number of adults admitted to hospital rather than number of admissions per adult). However, if exacerbations were reported as rate ratios, we analysed them on this basis.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when we identified a study as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic and visual inspection of the forest plots to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
As we were unable to pool more than 10 studies, we could not create and examine a funnel plot to explore possible small‐study and publication biases as planned in the protocol.
Data synthesis
We used a random‐effects model for all analyses, as we expected variation in effects due to differences in study populations and methods. We performed sensitivity analyses with a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table presenting data for all prespecified outcomes (Table 1). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), utilising GRADEpro software (GRADEpro GDT 2016). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for the primary outcomes:
individual versus group CBT;
mean age (18 years and younger versus older than 18 years);
baseline psychological symptoms (populations required to meet criteria for anxiety or depression versus populations with subclinical symptoms);
types of CBT (e.g. classic versus newer models)*.
We used the formal test for subgroup interactions in Review Manager 2014.
*We included third‐wave cognitive behavioural approaches in the scope of this review, but recognise that there are differences between these models and classic CBT, particularly in the way unhelpful thoughts are dealt with, which may lead to different outcomes.
In Table 2 we have presented key characteristics of the study populations and interventions to display other potential sources of heterogeneity that may not be easily assessed in subgroups (e.g. measures of asthma severity, concomitant use of asthma and psychotropic medications, frequency and duration of CBT sessions).
1. Summary of study characteristics.
| Study | N | Country (centres) | Asthma | Psychology | CBT | Outcome time points | Format | Mean age |
| Deshmukh 2008 | 18 | Australia (unclear) | NR | "with anxiety" | 4 sessions | 1.2 months EoT 3 months FU |
Group | NR |
| Grover 2002 | 10 | India (1) | NR | NR | 15 sessions | Unclear | Individual | NR |
| Grover 2007 | 40 | India (1) | 2+ years diagnosis | Those medicated or with psychiatric history excluded. | 15 sessions of 1 h | 1.5 to 2 months EoT | Individual | NR |
| Parry 2012 | 94 | UK (16) | "clinical diagnosis" | "highly anxious" as per HADS‐A or ASC‐PF cutoffs | 1.5 h intro 4 to 6 sessions of 1 hour ± 2 follow‐up sessions |
1.5 to 3 months EoT 6 months FU |
Individual | 43.4 |
| Pbert 2012 | 83 | USA (1) | NIH/NHLBI mild‐severe persistent | Those medicated or with psychiatric history excluded. | 8 sessions of 2.5 hours + 6‐hour session | 2.5 months EoT 6 and 12 months FU |
Group | 52.7 |
| Put 2003 | 23 | Belgium (1) | Diagnosis for at least 6 months | NR | 6 sessions of 1 hour | 3 months EoT 6 months FU |
Individual | 45.5 |
| Ross 2005 | 48 | Canada (unclear) | Under specialist care/recent attack | Panic disorder diagnosis, 3 recent attacks | 12 sessions of 1.5 hours | 2 months EoT 6 months FU |
Group | 39.0 |
| Sommaruga 1995 | 40 | Italy (1) | Diagnosed, treated, and followed up according to ATS guidelines | NR | 6 educational sessions (2 in and 4 out of hospital), 3 CBT sessions + 6 physician visits | 12 months after discharge | Individual | 47.5 |
| Yorke 2013 | 51 | UK (2) | Severe refractory asthma (ATS 2000) and BTS Steps 4 and 5 care | HADS anxiety or depression > 8 | 8 sessions of 1.5 h | 4 months FU | Group | NR |
ASC‐PF = Asthma Symptom Checklist Panic‐Fear subscale; ATS = American Thoracic Society; BTS = British Thoracic Society; CBT = cognitive behavioural therapy; EoT = end of treatment; FU = follow‐up; HADS‐A = Hospital Anxiety and Depression Scale, Anxiety scale; NIH/NHLBI = National Institutes of Health/National Heart, Lung, and Blood Institute; NR = not reported
Sensitivity analysis
We planned the following sensitivity analyses:
studies at high risk of bias for blinding of outcome assessors;
unpublished data (from conference abstracts or obtained from authors).
Results
Description of studies
Results of the search
We identified 740 records through database searching and 24 additional records by searching the WHO trials portal (n = 9), ClinicalTrials.gov (n = 13), and reference lists of included studies and existing systematic reviews (n = 4). We removed four duplicates, and screened the titles and abstracts of the remaining 760 unique records for inclusion. We excluded 701 on the basis of the titles and abstracts alone, and retrieved full papers for the remaining 59. Upon closer inspection of the papers, we found that 42 did not meet the inclusion criteria for the review (reasons given in Excluded studies and Figure 2), and recorded three of the records retrieved from trial registries as ongoing studies. We have included nine studies with 14 associated citations in the review, eight of which contributed to at least one meta‐analysis.
2.
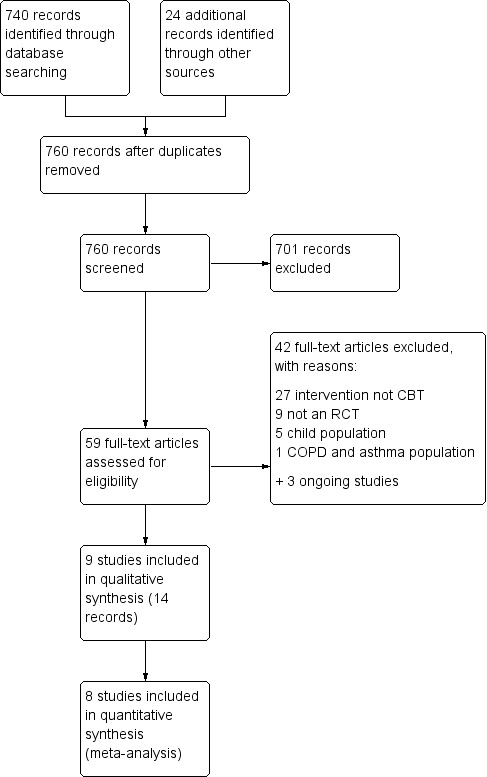
Study flow diagram.
Included studies
We identified nine studies (including 407 participants) that met the inclusion criteria for this review, with a total of 14 associated publications or reports. All of the studies were considered to be randomised controlled trials, although in some of the older trials the methods of selection and allocation were less clearly described. The studies were published between 1995 and 2013; two were only available as conference abstracts at the time of the writing of this review (Deshmukh 2008; Grover 2002). The studies were all relatively small, with a population size ranging from 10 to 94 (median 40). Four studies were conducted in Europe (Parry 2012; Put 2003; Sommaruga 1995; Yorke 2013), two in North America (Pbert 2012; Ross 2005), two in India by the same research team (Grover 2002; Grover 2007), and one in Australia (Deshmukh 2008). A summary of study characteristics is presented in Table 2, and more detailed descriptions are available in the Characteristics of included studies tables.
Participants
All studies recruited participants with asthma, either according to a physician's judgement or guideline‐defined criteria. Grover 2007 required a diagnosis for at least two years, and Put 2003 for six months with recent symptoms. Pbert 2012 required participants to meet criteria for mild, moderate, or severe persistent asthma according to National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHBLI) criteria. Ross 2005 and Yorke 2013 set criteria to recruit a more severe population, the former by requiring a referral to a pulmonary specialist and a recent emergency department visit for an exacerbation, and the latter by specifying that participants should meet criteria for severe refractory asthma (ATS 2000), and by recruiting participants from national specialist severe asthma clinics.
Three studies specifically recruited participants with psychological symptoms, although with very different criteria (Deshmukh 2008; Parry 2012; Yorke 2013). Deshmukh 2008 required "comorbid anxiety and asthma"; Parry 2012 and Yorke 2013 recruited people who met cutoffs on a psychological symptom scale; and Ross 2005 specifically recruited people with asthma and a primary diagnosis of panic disorder. Some studies excluded people with severe psychiatric illness (Parry 2012; Ross 2005; Yorke 2013), and others for a history of psychological illness requiring current or past use of psychotropic medication (Grover 2007; Parry 2012), a recent dose change (Ross 2005; Yorke 2013), or previous participation in a psychological or an educational intervention (Grover 2007; Parry 2012; Put 2003).
Studies specifying age recruited adults over 18, and mean age of the randomised populations ranged from 39.0 to 52.7. Most studies excluded some medical comorbidities, usually including at least other respiratory illnesses, but often cardiovascular disease and drug, alcohol, or nicotine dependence. No studies mentioned recruiting adolescents under the age of 18.
Minimal information about baseline characteristics or inclusion and exclusion criteria was available for Deshmukh 2008, Grover 2002, and Sommaruga 1995.
Interventions and comparisons
As per the eligibility criteria for this review, all of the studies tested a psychological intervention including cognitive and behavioural elements, although these varied in nature, duration, and delivery. Eight studies used a classic model of CBT (five individual, two group, and one unclear), and one used a group mindfulness‐based model (Pbert 2012). Where they were described, specific components of classic CBT could usually be categorised under asthma education, psycho‐education, relaxation or breathing techniques, cognitive restructuring, problem‐solving, and coping skills. Four studies did not describe the qualifications of those delivering the intervention (Deshmukh 2008; Grover 2002; Grover 2007; Pbert 2012). In the other five studies, the intervention was delivered by trained clinical psychologists (Put 2003; Sommaruga 1995; Yorke 2013), doctoral nurse clinicians (one trained in CBT and one as an asthma educator) (Ross 2005), or a mix of trained psychologists and a cognitive behavioural therapist (Parry 2012).
Six studies provided one‐on‐one sessions of classic CBT (Grover 2002; Grover 2007; Parry 2012; Put 2003; Sommaruga 1995); Grover 2002 and Grover 2007 both tested a 15‐session individual CBT program, although the earlier study used a standard pharmacotherapy control group, and the later one tested CBT on top of an asthma self management program compared to self management alone. The intervention in Parry 2012 consisted of 4 to 6 individual sessions over 6 to 13 weeks plus an introductory session, compared with a no‐treatment control group who were offered the intervention at the end of the study. Put 2003 gave six one‐hour individual sessions of classic CBT compared with a waitlist control group. The intervention in Sommaruga 1995 was described as an "Asthma Rehabilitation Group", which included three individual sessions of CBT as well as an educational programme, telephone access to the physician, daily peak flow monitoring, and a personal medication plan. The control group did not receive the educational programme or CBT and were treated according to guidelines and followed up six times during the yearlong study.
Three studies provided classic CBT in a group format (Deshmukh 2008; Ross 2005; Yorke 2013). Deshmukh 2008 tested a five‐week group cognitive behavioural intervention (four sessions) against an asthma‐monitoring control group, although the content of the sessions was unclear. The CBT model used in Ross 2005 was derived from Barlow panic control treatment and Beck cognitive treatment for panic disorder, and consisted of 12 90‐minute group sessions over eight weeks, compared with a waitlist control group who were offered the intervention after the study. Yorke 2013 administered eight 90‐minute group sessions of CBT based on a manual (Antoni 2003), and the control group received usual care.
One study integrated participants in the intervention group into mindfulness‐based stress reduction (MBSR) group sessions and offered control group participants a "healthy living course" with the same amount of contact (eight‐weekly 2.5‐hour sessions plus a 6‐hour session on week six) (Pbert 2012). MBSR included body scan, sitting meditation with a focus on breathing awareness, thoughts, and feelings; gentle stretching exercise, emphasising integration into everyday life to support coping with symptoms and stress; and CD‐based mindfulness exercises for home practice.
We investigated intervention format (individual or group sessions) and the model of CBT with planned subgroup analyses. Additional variation among studies in session number and length, and the type of control group makes some of the results more difficult to interpret; we have commented on this in the Discussion.
Outcomes
The studies generally measured similar types of outcomes, but the scales and definitions used varied considerably, particularly with regard to psychological symptoms.
In terms of asthma outcomes, all studies except Sommaruga 1995 measured quality of life, mostly with the Asthma Quality of Life Questionnaire (AQLQ), used in Deshmukh 2008, Grover 2002, Grover 2007, Pbert 2012, Put 2003, Ross 2005, and Yorke 2013 (Juniper 1999), but also with the Asthma Bother Profile, used in Grover 2007 and Parry 2012 (Hyland 1995), or general measures such as the EQ‐5D, used in Parry 2012 and Yorke 2013. Deshmukh 2008 data were calculated from individual participant data on a poster graph provided by the study authors. Measures of asthma symptoms and control included the Asthma Symptom Checklist (ASC), used in Grover 2002, Grover 2007, Parry 2012, Put 2003, and Sommaruga 1995 (Brooks 1989), often including the panic‐fear subscale as a measure of asthma‐related anxiety; the Asthma Control Questionnaire (ACQ) and Dyspnoea‐12, both used in Yorke 2013 (Juniper 1999a; Yorke 2011); NIH/NHLBI asthma control categorisations, used in Pbert 2012 (NIH/NHLBI 2007); and non‐validated measures including asthma diary data such as rescue medication use, peak flow, and symptom‐free days (Grover 2002; Grover 2007; Pbert 2012; Ross 2005; Yorke 2013). Five studies measured peak expiratory flow (Grover 2002; Grover 2007; Pbert 2012; Put 2003; Ross 2005). Other outcomes measured emotions and attitudes relating to asthma such as asthma‐related emotional functioning (Deshmukh 2008), health locus of control (Parry 2012; Sommaruga 1995), Knowledge, Attitude, and Self‐Efficacy Asthma Questionnaire, used in Put 2003, and the Respiratory Illness Opinion Survey (cited in Sommaruga 1995 through personal communication) (Wigal 1993).
In terms of psychological outcomes, anxiety was measured by the Hospital Anxiety and Depression Scale used in Deshmukh 2008, Grover 2007, Parry 2012, and Yorke 2013 (Zigmond 1983), State‐Trait Anxiety Inventory, used in Grover 2002 and Sommaruga 1995 (Spielberger 1983), and panic‐specific outcomes were measured in Ross 2005 due to the comorbid population. Depression was measured by the Beck Depression Inventory, used in Grover 2002 and Ross 2005 (Beck 1961), Negative Emotionality Scale, used in Put 2003 (Tellegen 1988), and Depression Questionnaire, used in Sommaruga 1995 (Sanavio 1986). Other psychological outcomes included the Perceived Stress Scale, used in Pbert 2012 (Cohen 1983), a semi‐structured interview schedule, used in Grover 2007, and the Anxiety Sensitivity Index, used in Ross 2005 (Peterson 1992). Put 2003 also measured adherence, and Yorke 2013 was the only study to measure acceptability of the intervention.
Excluded studies
After viewing the full‐text publications we excluded 42 studies. The most common reason for exclusion was that the intervention did not meet the inclusion criteria of CBT. We excluded nine studies because they were not randomised controlled trials, five studies because they recruited child populations, and one study because the population included people with asthma or COPD. It was difficult to ascertain the nature of interventions from abstracts alone, and even from the full‐texts, especially when the intervention included cognitive and behavioural elements but was not described as CBT. This led to several discussions regarding inclusion and the application of the eligibility criteria, and a large number of excluded studies to properly document this process.
In addition to the excluded studies, we listed three studies as ongoing (ACTRN12614000915651; IRCT2015061622770N1; NCT01583296). ACTRN12614000915651 is an Australian trial of telephone‐delivered CBT and will include participants with asthma and other lung diseases undergoing pulmonary rehabilitation (COPD, idiopathic pulmonary fibrosis, bronchiectasis), so it will only be eligible for inclusion in a future update if disaggregated data are made available. The authors of this study aim to recruit 100 participants, but the study, which was due to start in September 2014, is listed as "not yet started recruiting". IRCT2015061622770N1 is a study of mindfulness‐based cognitive therapy for women with asthma, evaluating its effect on anxiety, depression, and somatic symptoms. The study is being conducted in Iran, was registered in December 2015, and aims to recruit 30 participants. NCT01583296 has the acronym LUCHAR and is listed as completed, but currently has no listed publications or data posted on ClinicalTrials.gov. It is a study of CBT with heart rate variability feedback versus Music Relaxation Therapy (MRT), and so may not meet the inclusion criteria for this review since it has an active comparison. The study is being conducted in New York, USA and aimed to enrol 53 Latino participants.
Risk of bias in included studies
A summary of the risk of bias across studies is presented in Figure 3.
3.
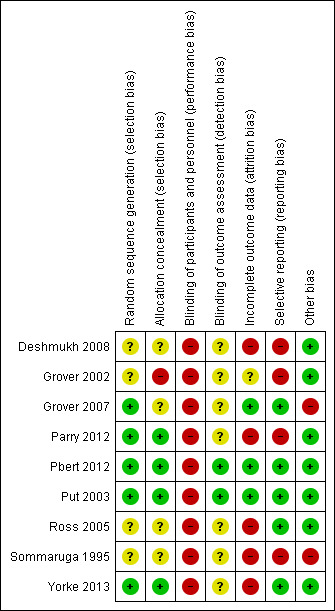
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered four studies to be at low risk of bias for both random sequence generation and allocation concealment because they reported adequate methods in the published reports (usual computerised schedules implemented centrally) (Parry 2012; Pbert 2012; Put 2003; Yorke 2013). We rated three studies as unclear for both domains because they were described as randomised but with insufficient details about methods to make a judgement about possible bias (Deshmukh 2008; Ross 2005; Sommaruga 1995). We rated Grover 2002 as at unclear risk for random sequence generation for the same reason, and high risk for allocation concealment because the report stated that participants were "sequentially allotted to two groups", which could have allowed for bias in implementation of the sequence. We rated Grover 2007 as at low risk for sequence generation because a random number table was used, but unclear for allocation concealment because no other details were given.
Blinding
The behavioural nature of the interventions of interest in this review could not be kept blind from participants and personnel. As a result, we rated blinding of participants and personnel as high risk of bias by default. However, when rating each outcome in GRADE, we considered the differential effect performance bias was likely to have had on subjective outcomes including self rated questionnaires and objective outcomes such as exacerbations and adverse events.
Regardless of the inability to blind participants and personnel, it was possible to reduce bias for all outcomes by recruiting someone not otherwise involved in the study to measure outcomes without knowledge of allocation. We did not assume this was done and rated studies high risk by default unless it was explicitly stated in the study report or via personal communication.
Incomplete outcome data
We considered five studies to be at high risk of attrition bias: Deshmukh 2008 included very low numbers in each group and saw very high and unbalanced dropout; in Parry 2012, there was very high and unbalanced dropout (60% and 35% for intervention and control), which is unlikely to have been fully accounted for by the imputation for the intention‐to‐treat model; demographic and outcome data in Ross 2005 were reported for the subset of participants who completed, which was only 52% of those who were randomised; we considered Sommaruga 1995 as at high risk as no participants dropped out of the intervention group, whereas 20% of the control group dropped out during follow‐up; in Yorke 2013, seven participants were removed after randomisation, which may have biased the results, and there was a large amount of missing data from the asthma diaries due to poor adherence.
Attrition bias was unclear in Grover 2002 because only a conference abstract was available, and we considered the remaining studies as at low risk of attrition bias, either because there was no dropout, because dropout was relatively low and balanced between groups, or because imputation is likely to have appropriately accounted for missing data.
Selective reporting
We rated four studies as at high risk of bias for selective reporting, two of which were only available as conference abstracts, so very little information was available regarding the conduct of the study or the results (Deshmukh 2008; Grover 2002). We also rated Parry 2012 and Sommaruga 1995 as at high risk because some results were only reported as "no significant difference" or at baseline and not after treatment.
Risk of reporting bias was considered for each outcome separately in the GRADE process, so a high‐risk rating does not affect our grading of other unrelated outcomes.
We rated the other five studies as at low risk of bias, either because we were able to check the reported outcomes against a prospectively registered protocol (Pbert 2012), or because outcomes listed in the methods were fully reported in a way that allowed data to be included in our analyses (Grover 2007; Put 2003; Ross 2005; Yorke 2013).
Other potential sources of bias
We considered two studies to be at high risk of bias for another reason: Sommaruga 1995 because the intervention group received additional interventions, which may have confounded the result, and Grover 2007 because there were baseline imbalances across groups for the Asthma Bother Profile, ASC, and Hospital Anxiety and Depression Scale. We rated the other seven studies as at low risk of bias because no other biases were noted.
Effects of interventions
See: Table 1
Asthma‐related quality of life
Six studies reported asthma‐related quality of life on the AQLQ (Deshmukh 2008; Grover 2007; Pbert 2012; Put 2003; Ross 2005; Yorke 2013), showing a 0.55‐point benefit of CBT over usual care at the end of treatment (95% confidence interval (CI) 0.17 to 0.93; Analysis 1.1). The primary endpoint measurements were taken between 5 and 16 weeks, depending on the length of treatment across studies. As planned in our protocol, where available we used change from baseline measurements. We considered the evidence to be of low quality due to possible performance and attrition bias, and variation between study results (I2 = 53%, P = 0.06). We removed Deshmukh 2008 in a sensitivity analysis because there was very high attrition in the control group (leaving only three participants in that arm), and the data were estimated from a poster graph. The magnitude of the effect based on the remaining five studies was slightly smaller but still statistically significant in favour of CBT (mean difference (MD) 0.48, 95% CI 0.07 to 0.89).
1.1. Analysis.
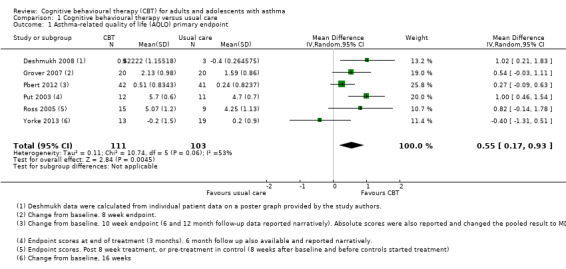
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 1 Asthma‐related quality of life (AQLQ) primary endpoint.
Follow‐up data were available at 3 months for Deshmukh 2008, 6 and 12 months for Pbert 2012, and 6 months for Put 2003; all showed a statistically significant effect of CBT over usual care on the AQLQ (Analysis 1.2).
1.2. Analysis.
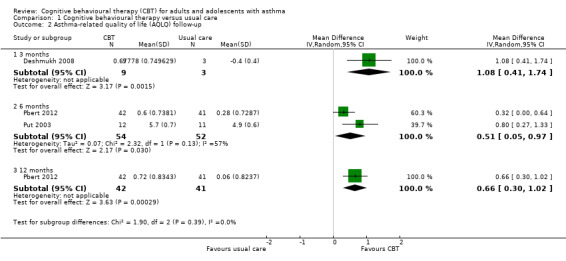
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 2 Asthma‐related quality of life (AQLQ) follow‐up.
Deshmukh 2008 also reported the number of participants showing an important improvement on the AQLQ (i.e. meeting the scale's minimal clinically important difference (MCID) of 0.5 from baseline to end of treatment). The numbers were small, and only 3 of the 8 participants in the control group could be followed up, but the study reported that 6 out of 9 and 5 out of 9 in the CBT group met the MCID at the end of treatment and 3‐month follow‐up, and nobody in the control group.
Asthma exacerbations requiring at least a course of oral steroids
Parry 2012, Pbert 2012, and Yorke 2013 reported outcomes that could be interpreted as asthma exacerbations, but in very different ways, so that they could not be meta‐analysed.
Parry 2012 reported the number of participants in the six months before treatment (but not in the period afterwards) that had been admitted to hospital, which we have summarised in the unscheduled contacts outcome below. At post‐treatment (10 weeks) and at the 6‐ and 12 month follow‐ups, Pbert 2012 reported the number of participants who had recently had a course of prednisolone (within 30 days of measurement), but there were important differences in recent predinisolone use at baseline (10 out of 41 CBT and 2 out of 41 control) so it was difficult to interpret the results; 5, 5, and 7 participants out of 39 in the CBT group had recently had a course of prednisolone at 10‐weeks (post‐treatment), 6‐months and 12‐months, compared to 6, 2, and 7 participants in the control group, respectively. Two participants in the CBT group and 1 in the control group of Yorke 2013 recorded an emergency department or hospital visit for an exacerbation, but this was based on a subset of 7 participants in each group with complete diary card data.
We did not GRADE the quality of this evidence.
Asthma control
Three studies reported validated scales of asthma control, either the ASC, in Grover 2007 and Put 2003, or the ACQ, in Yorke 2013. The pooled result showed an overall benefit of CBT over usual care (standardised mean difference (SMD) ‐0.98, 95% CI ‐1.76 to ‐0.20; participants = 95; Analysis 1.3), although there was significant variation among the study results (I2 = 68%, P = 0.04). We downgraded the evidence once for risk of performance and attrition bias and once for inconsistency, and rated it low quality.
1.3. Analysis.
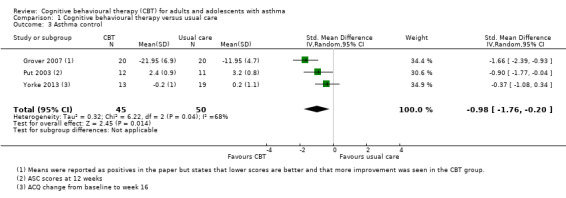
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 3 Asthma control.
In addition to the validated scales pooled in the analysis, we noted the following outcomes relating to asthma control.
CBT did not reduce the need for rescue medication use per week in Pbert 2012, ranging from 2.39 to 3.21 times across the three time points in the CBT group (10 weeks, 6 months, and 12 months) and from 1.83 to 2.49 in the control group.
In the same study, the number of participants meeting NIH/NHLBI criteria for 'well‐controlled' was similar at the 10‐week endpoint (3/33 CBT and 5/37 control), but showed a possible longer‐term benefit of CBT at the 6‐month (8/37 CBT and 2/37 control) and 12‐month follow‐up (7/36 CBT and 3/38 control).
In Ross 2005, the number of symptom‐free days over two weeks was similar in the CBT (6.69, standard deviation (SD) 5.72) and control groups (5.62, SD 4.98), based on 13 and 8 participants in the two groups after 8 weeks, respectively.
Unscheduled contacts with health services for asthma
Data about unscheduled contact was not generally reported, or not in a way that could be meta‐analysed. Parry 2012 reported data as the mean number of visits per participant over the six months after treatment (Analysis 1.4), and did not find a difference between CBT and control participants for general practitioner visits (MD ‐0.28, 95% CI ‐1.36 to 0.80) or primary care visits including nurse and out‐of‐hours contacts (MD ‐0.40, 95% CI ‐1.51 to 0.71). We considered evidence for these outcomes to be of low quality because the study was at high risk of performance and attrition bias (risk of bias downgrade), and because the effects were based on data from one study of 80 participants (imprecision downgrade).
1.4. Analysis.
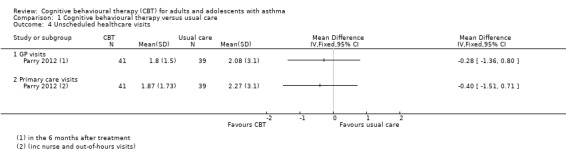
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 4 Unscheduled healthcare visits.
Otherwise, as stated under the exacerbation outcome, 2 participants in the CBT group and 1 in the control group of Yorke 2013 recorded an emergency department or hospital visit for an exacerbation, but this was based on incomplete diary card data. Parry 2012 reported that 3 participants in the CBT group and 4 in the control group were admitted to hospital in the six months before treatment, but the numbers in each group were unclear, and the equivalent poststudy data were not reported.
Validated scales of anxiety
We were unable to pool all anxiety data due to variation in the scales and analyses used. We analysed studies in three unpooled subgroups for anxiety measured as:
change from baseline (Parry 2012; Pbert 2012; Yorke 2013);
anxiety as endpoint scores (Parry 2012; Ross 2005; Sommaruga 1995); and
anxiety scores as a composite with depression (Grover 2007; Yorke 2013).
These were presented as subgroups in one analysis (Analysis 1.5), but the change scores were our primary analysis, as defined in our protocol (Kew 2015). These could not be combined in a SMD analysis, as the smaller change from baseline variances would have given those studies more weight in the analysis.
1.5. Analysis.
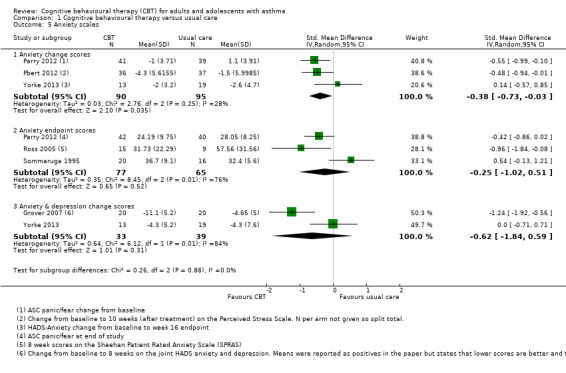
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 5 Anxiety scales.
Studies reporting change from baseline showed that CBT improved anxiety scores compared with usual care (SMD ‐0.38, 95% CI ‐0.73 to ‐0.03), but this was not backed up by the endpoint scores analysis (SMD ‐0.25, 95% CI ‐1.02 to 0.51). There was significant variation between study results in the endpoint score (I2 = 76%, P = 0.01), but not in the change scores (I2 = 28%, P = 0.25). We primarily graded the change score analysis, but took the endpoint analysis into consideration. We considered the evidence to be of low quality due to possible performance and attrition bias (risk of bias downgrade) and inconsistency between study results and the two analyses (inconsistency downgrade).
Two studies reported change in the total Hospital Anxiety and Depression Scale (HADS) anxiety and depression composite score (Grover 2007; Yorke 2013). The studies showed very different effects, which made the result difficult to interpret (SMD ‐0.62, 95% CI ‐1.84 to 0.59; I2 = 84%).
Validated scales of depression
Similarly to the anxiety outcomes, some studies reported depression scales as change from baseline (Parry 2012; Yorke 2013), and others as endpoint scores (Put 2003; Ross 2005; Sommaruga 1995), which could not be pooled in a SMD analysis. The pooled result from two studies reporting depression as change from baseline, both using the HADS (SMD ‐0.33, 95% CI ‐0.70 to 0.05), was similar to the pooled result of the three studies reporting endpoint scores (SMD ‐0.41, 95% CI ‐0.87 to 0.05); neither upper confidence intervals ruled out no difference between CBT and usual care. There was significant heterogeneity between the endpoint scores (I2 = 80%, P = 0.007), which may be due to each study using different scales and time points (Negative Emotionality Scale at 3 months in Put 2003, Beck Depression Inventory at 8 weeks in Ross 2005, and Depression Questionnaire in Italian at 52 weeks in Sommaruga 1995). As with the anxiety analyses, we primarily graded the change score analysis, as this preference was stated in our protocol, but we took the endpoint analysis into consideration. We downgraded the evidence for publication and attrition bias (risk of bias downgrade) and imprecision in the estimate (imprecision downgrade).
Put 2003 also measured the Negative Emotionality Score at 6‐month follow‐up, showing a similar but slightly smaller effect of CBT than at the 3‐month post‐treatment measurement.
Medication adherence
Only one study used the Adherence Scale (Put 2003), on which higher scores indicate poorer adherence. The mean score was lower in the CBT group than in the usual care group, but the confidence intervals for the effect did not exclude no difference between groups (MD ‐1.40, 95% CI ‐2.94 to 0.14; participants = 23; studies = 1; I2 = 0%). Mean scores were similar in both groups at 6‐month follow‐up. We downgraded the evidence twice for imprecision due to the very small number of participants in the analysis and uncertainty in the effect, and rated it low quality.
Parry 2012 reported the number of prescriptions taken 6 months before (1.53, SD 0.92 (CBT); 1.43, SD 1.40 (usual care)), during (1.33, SD 1.40 (CBT); 1.32, SD 1.10 (usual care)), and 6 months after treatment (1.27, SD 1.30 (CBT); 1.00, SD 1.30 (usual care)), showing a slight reduction over time in both groups and no real differences between them. It is unclear whether this outcome was a measure of adherence to treatment (higher is better) or the number of different prescriptions required for asthma control (lower is better).
Subgroup analyses
We planned to conduct subgroup analyses on the three primary outcomes: asthma‐related quality of life, exacerbations requiring at least a course of oral steroids, and asthma control. While we did not specify a minimum number of studies needed to conduct the subgroup analyses, only three studies contributed data to the second and third primary outcomes, which we did not consider to be sufficient for subgroup analyses. As such, we conducted subgroup analyses on the asthma‐related quality of life outcome only. The observational nature of subgroup analyses, along with the small number of studies and variation between their designs, populations, and other intervention characteristics, limited our confidence in the subgroup analyses.
Individual versus group CBT
Of the six studies reporting the AQLQ, two used an individual CBT format (Grover 2007; Put 2003), and four used a group format (Deshmukh 2008; Pbert 2012; Ross 2005; Yorke 2013). There was some heterogeneity within both subgroups, and the test for subgroup differences was not statistically significant (I2 = 11%, P = 0.29).
Mean age
We were unable to make the comparison of adolescents (younger than 18 years) and adults because all of the included studies recruited adult populations.
Baseline psychological symptoms
The results of the three studies recruiting populations with evident psychological symptoms at baseline varied widely among studies (I2 = 65%, P = 0.06) (Deshmukh 2008; Ross 2005; Yorke 2013), which meant that the subgroup effect had extremely wide confidence intervals. Studies that did not recruit participants on the basis of psychological symptoms also varied significantly within the subgroup (I2 = 60%, P = 0.08). The test for differences between the two subgroups was not significant (I2 = 0%).
Types of CBT
As with the other subgroup analyses, variation within the subgroups outweighed differences between them. There was much heterogeneity (I2 = 49%, P = 0.10) among the five studies using a classic CBT model (Deshmukh 2008; Grover 2007; Put 2003; Ross 2005; Yorke 2013), and the test for subgroup differences between these studies and the one study using a mindfulness model, Pbert 2012, was not significant (I2 = 38%, P = 0.20).
Sensitivity analyses
Studies at high risk of bias for blinding of outcome assessors
We rated none of the studies as at high risk for detection bias. We rated two studies as at low risk (Pbert 2012; Put 2003), and we did not know whether outcome assessors were blind in the rest.
For the first primary outcome, AQLQ, limiting the analysis to the two studies rated as at low risk did not have a large impact on the point estimate (MD 0.61), but the confidence intervals were much wider (95% CI ‐0.11 to 1.32), and there was inconsistency between the two results (I2 = 80%, P = 0.03).
Only one of the low‐risk studies, Put 2003, appeared in the second primary outcome analysis for asthma control, and the effect for this study alone (SMD ‐0.90, 95% CI ‐1.77 to ‐0.04) was similar to the pooled result for all three in the analysis (SMD ‐0.98, 95% CI ‐1.76 to ‐0.20).
We were unable to perform a meta‐analysis for the third primary outcome, exacerbations requiring oral steroids, so it did not make sense to do a sensitivity analysis.
Unpublished data
We calculated Deshmukh 2008 AQLQ data from a graph on a poster provided by the study authors. These data were not available in the associated abstract, and the study has not been fully published. In addition, calculating mean change scores from the bar graph of baseline, endpoint, and follow‐up scores of each participant involved some measurement error and imprecision. When we removed these data from a sensitivity analysis from the primary endpoint, the magnitude of the effect was slightly smaller, but still statistically significant in favour of CBT (MD 0.48, 95% CI 0.07 to 0.89).
No unpublished data contributed to the other two primary outcomes, asthma control and exacerbations requiring oral steroids.
Discussion
Summary of main results
We found nine randomised trials including 407 adults with asthma. Study size ranged from 10 to 94 (median 40), and mean age ranged from 39 to 53. Study populations generally had persistent asthma, but severity and diagnostic measures varied. Three studies recruited participants with a psychological symptomatology, although with very different criteria.
Most studies used a classic model of CBT, given either individually, in Deshmukh 2008, Grover 2002, Grover 2007, Parry 2012, and Put 2003, or in a group (Ross 2005; Yorke 2013), and one study tested a group mindfulness intervention (Pbert 2012). Interventions ranged from 4 to 15 sessions, and primary measurements were taken at a mean of 3 months (range 1.2 to 12 months), and there was also variation in the control groups. Studies generally measured similar outcomes, but the scales and definitions used varied considerably, particularly with regard to psychological symptoms. The inability to blind participants and investigators to group allocation introduced a serious potential for bias, and high dropout was also an issue in some studies. Evidence quality was low, often affected by these risks of bias in combination with either imprecision or inconsistency between study results.
Participants given CBT had improved scores on the AQLQ (MD 0.55, 95% CI 0.17 to 0.93; participants = 214; studies = 6; I2 = 53%) and on measures of asthma control (SMD ‐0.98, 95% CI ‐1.76 to ‐0.20; participants = 95; studies = 3; I2 = 68%) compared to participants getting usual care. The AQLQ effect appeared to be sustained up to a year after treatment, but all of the evidence must be interpreted with caution due to the low quality of the evidence. Asthma exacerbations requiring at least a course of oral steroids were not consistently reported, so we could not perform a meta‐analysis.
Data were generally sparser for the secondary outcomes. One study of 80 participants that could be analysed for unscheduled contacts did not show a difference between CBT and usual care for general practitioner visits (MD ‐0.28, 95% CI ‐1.36 to 0.80) or primary care visits including nurse and out‐of‐hours contacts (MD ‐0.40, 95% CI ‐1.51 to 0.71) (Parry 2012). Anxiety scores were difficult to pool but showed a benefit of CBT compared with usual care (SMD ‐0.38, 95% CI ‐0.73 to ‐0.03), although this depended on the analysis used. The confidence intervals for the effect on depression scales included no difference between CBT and usual care when measured as change from baseline (SMD ‐0.33, 95% CI ‐0.70 to 0.05) or endpoint scores (SMD ‐0.41, 95% CI ‐0.87 to 0.05), and the same was true for medication adherence (MD ‐1.40, 95% CI ‐2.94 to 0.14; participants = 23; studies = 1; I2 = 0%).
Subgroup analyses conducted on the AQLQ outcome did not suggest a clear difference between individual and group CBT, baseline psychological status, or CBT model. The small number of studies and the variation between their designs, populations, and other intervention characteristics limited the conclusions that could be drawn about these possibly moderating factors.
Overall completeness and applicability of evidence
Several factors warrant consideration when interpreting the completeness and applicability of the present findings. The search strategy was designed to identify interventions that included CBT as the main active component. The nine studies included in this review mostly used a classic CBT model, although the delivery of the intervention varied (including individual and group therapy), and the number and duration of CBT sessions was mixed. There was patchy detail across studies about the actual content of CBT, who had delivered the intervention, intervention fidelity, and possible contamination during the course of the study, making replication and application of the results difficult.
We specified 'usual care' as the comparator of interest to keep the comparison as pure as possible, but control groups varied more than anticipated, which makes the results harder to interpret. In practice, the control groups varied, with descriptions including no treatment (Parry 2012), waiting list (Pbert 2012; Ross 2005), standard pharmacological care (Grover 2002), usual care (Yorke 2013), and asthma monitoring (Deshmukh 2008). Participants in the control group of Grover 2007 received a self monitoring programme, which the intervention group also received on top of CBT; given that the effects of the self monitoring programme would theoretically cancel out, this study fits our inclusion criteria. We were satisfied that the control groups across these seven studies received something akin to 'usual care', which would of course differ across study contexts and likely be more intensive than real‐life care, due to study assessments, etc., which is the case in any meta‐analysis of trials. The control groups in Pbert 2012 and Sommaruga 1995 were more complicated and may have introduced clinical heterogeneity into the analyses to which they contributed, particularly as Pbert 2012 used a third‐wave group mindfulness intervention that differed from the classic models used in the other studies. Pbert 2012 gave a "Healthy Living Course", which matched the contact of the intervention group to isolate the specific effects of CBT, and aspects of the CBT group in Sommaruga 1995 (peak flow measurements, access to physician, and asthma education) were not well controlled for in the control group, who were followed up more regularly than could be considered 'usual care' (six times over the course of the year). We considered the study comparisons to broadly match the eligibility criteria set out in our protocol, but were nonetheless cautious in our conclusions due to this variation.
Our seven predetermined outcomes were reported in at least one study. Our primary outcomes of asthma‐related quality of life and asthma exacerbations are relevant outcomes in asthma, however not all studies included these and often used different mechanisms to assess these outcomes. This made pooling of the data difficult, and we could perform meta‐analysis on six studies using the AQLQ (Deshmukh 2008; Grover 2007; Pbert 2012; Put 2003; Ross 2005; Yorke 2013). We set five secondary outcomes, which were measured in a variety of ways across different studies. Anxiety was assessed in six studies (Grover 2007; Parry 2012; Pbert 2012; Ross 2005; Sommaruga 1995; Yorke 2013), and depression was assessed in five studies (Parry 2012; Put 2003; Ross 2005; Sommaruga 1995; Yorke 2013), however variation in the scales used prevented meta‐analysis for these outcomes. The remaining three outcomes were reported less frequently, limiting our ability to conduct any meaningful meta‐analyses.
All 407 participants were reported to have a confirmed diagnosis of asthma, although the mechanism of diagnosis was not always clearly stated. Psychological symptomatology is especially relevant in severe asthma, and only one study specifically focused on this group (Yorke 2013). Three studies assessed psychological symptoms as inclusion criteria (Deshmukh 2008; Ross 2005; Yorke 2013), although each study applied a different method of assessment. Differences in the psychological symptomatology of the study populations raise an important question of who with asthma might best benefit from CBT, but this could not be teased out in this review due to the different inclusion criteria used and the number of studies. Additionally, all of the studies recruited adult populations, so we were unable to draw any conclusions relating to the efficacy of CBT in adolescent populations.
It is therefore not possible to draw any firm conclusions as to the efficacy of CBT in the management of asthma.
Quality of the evidence
We assessed all of the evidence presented in this review as of low quality, meaning "our confidence in the effect estimate is limited" and "the true effect may be substantially different from the estimate of the effect" (GRADEpro GDT 2016). We did not pool any data for one of the primary outcomes, asthma exacerbations requiring at least a course of oral steroids, and we did not attempt to GRADE the quality of the narrative data.
We downgraded evidence for four outcomes for risk of bias (asthma‐related quality of life, asthma control, and validated scales of anxiety and depression), primarily because the subjective nature of rating scales may have allowed for bias due to the inability to blind participants and personnel to group assignment. In addition, we considered high or unbalanced dropout to be an issue in five studies, which may have introduced further bias in the outcomes to which they contributed (Deshmukh 2008; Parry 2012; Ross 2005; Sommaruga 1995; Yorke 2013). We did not downgrade the evidence for the outcome 'unscheduled contacts with health services for asthma' because it was unclear whether knowledge of treatment allocation would have affected this outcome as it did the subjective rating scales. There was a risk of attrition bias for this outcome, so these issues are still worth considering, even though we did not consider them sufficient to warrant downgrading the evidence.
There was important variation between study results for three outcomes (asthma‐related quality of life, asthma control, and validated scales of anxiety), which led to downgrades. For quality of life and asthma control, the overall heterogeneity between study results was 53% and 58%, respectively, which was deemed statistically significant at the 0.10 level recommended for the test (Higgins 2011). For the anxiety scales, the variability in scales used meant we had to combine results using standardised mean difference, which prevented us pooling change scores with endpoint measurements. This made it difficult to assess overall heterogeneity across the outcome, but we chose to downgrade because there was important variation between the endpoint scores (I2 = 76%, P = 0.01) and inconsistency between the pooled effects depending on whether studies reported changes or endpoint measurements. We faced a similar dilemma for the validated scales of depression outcome but chose not to downgrade in that instance because while there was important variation between studies reporting endpoint scores, studies reporting change scores were consistent with each other, and the pooled effects for changes and endpoints were in agreement with each other.
Our confidence in the evidence for three of the outcomes was reduced by imprecision in the estimates (unscheduled contacts, validated scales of depression, and medication adherence). For two of these outcomes, unscheduled contacts and medication adherence, we could analyse only one study (Parry 2012 and Put 2003, respectively), and the small number of participants in the analyses led us to downgrade each of these outcomes twice for imprecision. It was not possible in either case to say with any certainty that CBT is likely to have any benefit, or indeed cause harm, compared with usual care. The imprecision in the depression analysis was less severe, but it still prevented us from ruling out the possibility that CBT is no better than usual care, so we downgraded the outcome once.
We did not downgrade any outcomes for indirectness of the evidence to the question we set out to answer in the systematic review. While some studies looked at more specific populations than others (e.g. Yorke 2013 recruited only people with severe asthma), none of the studies included participants or tested interventions that did not meet the inclusion criteria for this review. The intervention group in Sommaruga 1995 received additional interventions, which may have confounded the results in that study, but we did not deem this sufficient to downgrade the two outcomes to which it contributed (validated scales of anxiety and depression).
We did not downgrade any of the outcomes for publication bias because we did not strongly suspect in any case that unpublished data would have changed the effects we observed or our confidence in them.
Potential biases in the review process
As with any systematic review, there is an element of subjectivity when deciding what should and should not be pooled in a meta‐analysis, which was particularly relevant in this review due to the range of scales and analysis methods used across studies. We attempted to reduce any bias that might be associated with these decisions by following the published protocol (Kew 2015), and being transparent in describing narratively anything that we decided not to pool.
The author team expanded after the protocol was written, which allowed us to extract study characteristics in duplicate to reduce the potential for error. We also found a large number of potentially eligible studies that needed to be considered in more detail, and this led to a more lengthy duplicate process of consideration. As described in the protocol, we have logged all references that were considered in detail during this process as excluded studies with explanations of our rationale for not including them in the review. Otherwise, we did not make any changes to the protocol except where it was not possible to follow the protocol due to the number of studies, and we have recorded these in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
A previous Cochrane review investigated the effects of any psychological intervention for people with asthma (Yorke 2006). This review assessed 15 studies of 687 participants across a range of interventions (CBT, cognitive therapy, behavioural therapy, relaxation, biofeedback, and counselling), and was limited in conclusions that could be drawn from it by variation in the interventions studied, small studies, and incomplete reporting. Of the 15 included studies, the majority assessed a form of relaxation technique including hypnosis, functional or progressive relaxation, mental imagery, and autogenic training. An earlier non‐Cochrane review focusing on relaxation therapies found 15 randomised controlled trials (Huntley 2002), but neither review found evidence for efficacy of such techniques in asthma. Refining the scope of the Yorke 2006 review to shift the focus to CBT, we found nine studies of 407 participants, only three of which were included in Yorke 2006. The refined scope and the number of CBT trials conducted since 2006 have allowed this current review to make more focused conclusions, finding evidence that people with asthma given CBT may have improved scores on the AQLQ and improved asthma control and anxiety levels. However, both reviews rely on low‐quality evidence due mainly to possible internal biases and lack of precision, meaning further studies may still change the conclusions or our confidence in them.
We are not aware of other systematic reviews assessing the effect of CBT on psychological and asthma outcomes for people with asthma, but numerous Cochrane and non‐Cochrane systematic reviews have found benefits of CBT over no treatment for other physical conditions (e.g. Bernardy 2013; Martinez‐Devesa 2010; Monticone 2015; Price 2008). These reviews often have similar reservations to ours regarding the quality of evidence, often due to small trials. There is often disparity between benefits on psychological and condition‐specific outcomes, and asthma may be unique in this regard due to the overlap and interaction between breathing difficulties, hyperventilation, and panic. Other CBT reviews including head‐to‐head comparisons generally fail to show superiority of CBT over other psychological treatments (e.g. Monticone 2015), which we did not address in our review, and this may be a possible area for future investigation in asthma.
Authors' conclusions
Implications for practice.
For people with persistent asthma, CBT may improve quality of life, asthma control, and anxiety levels compared with usual care. Risks of bias, imprecision of effects, and inconsistency between results reduced our confidence in the results to low, and evidence was lacking regarding the effect of CBT on asthma exacerbations, unscheduled contacts with health services for asthma, depression, and medication adherence. There was much variation between studies in how CBT was delivered and what constituted usual care, meaning the most optimal method of CBT delivery, format, and target population requires further investigation. There is currently no evidence for the use of CBT for adolescents with asthma.
Implications for research.
Pooled effects suggest CBT may have modest benefits for people with asthma, but it remains unclear who is most likely to benefit, from what sort of programme, and whether CBT is superior to other psychological interventions. The evidence could be better applied by stratifying results by age, asthma severity, or scores on psychological scales within studies, and/or with head‐to‐head comparisons of different CBT formats and programmes to explore resource implications. The current evidence offers little insight into possible harms of CBT, which could be reported in more detail in studies of this nature, and evidence for younger populations is lacking.
Acknowledgements
We would like to thank Elizabeth Stovold for designing the search strategy for this project, and the rest of the Cochrane Airways Group for their support. We are very grateful for the information and unpublished data provided by Vandana Deshmukh.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
Kristin Carson was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE (Ovid) search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
Note: The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials in the Cochrane Airways Group Specialised Register
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Behavior Therapy Explode All
#6 MeSH DESCRIPTOR Psychotherapy
#7 CBT:TI,AB,KW
#8 cognitiv* NEAR3 (behav* or treatment* or technique* or therap* or intervention* or restructur* or reappraisal*)
#9 behav* NEAR3 (treatment* OR therap* or intervention* OR activat* or technique* or modif* or change*)
#10 coping* NEAR3 (skill* or strateg*)
#11 psychotherap*
#12 psychological*
#13 talk* NEAR3 (therap* or intervention*)
#14 anxiety or anxious*
#15 panic*
#16 stress*
#17 depress*
#18 mood*
#19 mindful*
#20 acceptance* NEAR commitment*
#21 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 OR #17 OR #18 OR #19 OR #20
#22 #4 and #21
[Note: In search line #1, MISC1 denotes the field in which the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Cognitive behavioural therapy versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Asthma‐related quality of life (AQLQ) primary endpoint | 6 | 214 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.17, 0.93] |
| 2 Asthma‐related quality of life (AQLQ) follow‐up | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 1.08 [0.41, 1.74] |
| 2.2 6 months | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.05, 0.97] |
| 2.3 12 months | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.30, 1.02] |
| 3 Asthma control | 3 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.76, ‐0.20] |
| 4 Unscheduled healthcare visits | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 GP visits | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Primary care visits | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Anxiety scales | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Anxiety change scores | 3 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.73, ‐0.03] |
| 5.2 Anxiety endpoint scores | 3 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.02, 0.51] |
| 5.3 Anxiety & depression change scores | 2 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.84, 0.59] |
| 6 Depression scales | 5 | Std. Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 Depression change scores | 2 | 112 | Std. Mean Difference (Fixed, 95% CI) | ‐0.33 [‐0.70, 0.05] |
| 6.2 Depression endpoint scores | 3 | 83 | Std. Mean Difference (Fixed, 95% CI) | ‐0.41 [‐0.87, 0.05] |
| 7 Medication adherence | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.94, 0.14] |
1.6. Analysis.
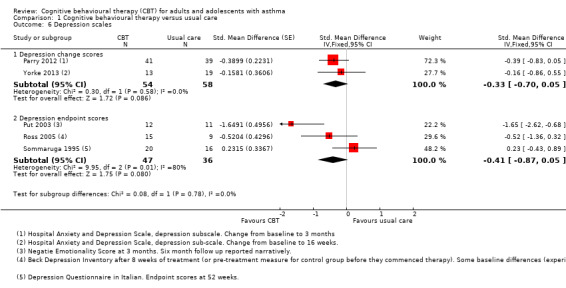
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 6 Depression scales.
1.7. Analysis.
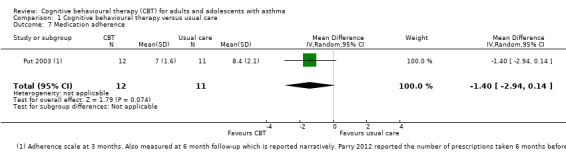
Comparison 1 Cognitive behavioural therapy versus usual care, Outcome 7 Medication adherence.
Comparison 2. Subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Individual vs group CBT: AQLQ | 6 | 214 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.17, 0.93] |
| 1.1 Individual | 2 | 63 | Mean Difference (IV, Random, 95% CI) | 0.78 [0.33, 1.23] |
| 1.2 Group | 4 | 151 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.11, 0.93] |
| 2 Baseline psychology: AQLQ | 6 | 214 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.17, 0.93] |
| 2.1 Psychological symptoms | 3 | 68 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.38, 1.36] |
| 2.2 No psychological symptoms | 3 | 146 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.13, 1.01] |
| 3 CBT models: AQLQ | 6 | 214 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.17, 0.93] |
| 3.1 Classic CBT | 5 | 131 | Mean Difference (IV, Random, 95% CI) | 0.64 [0.19, 1.10] |
| 3.2 MBSR | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.09, 0.63] |
2.1. Analysis.
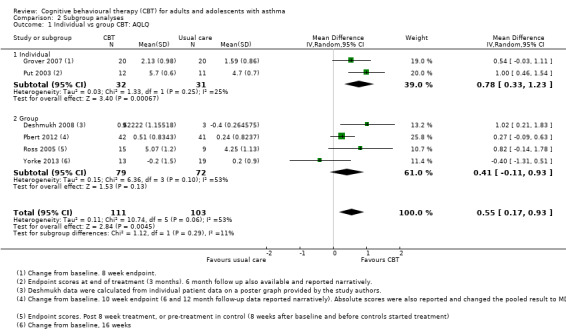
Comparison 2 Subgroup analyses, Outcome 1 Individual vs group CBT: AQLQ.
2.2. Analysis.
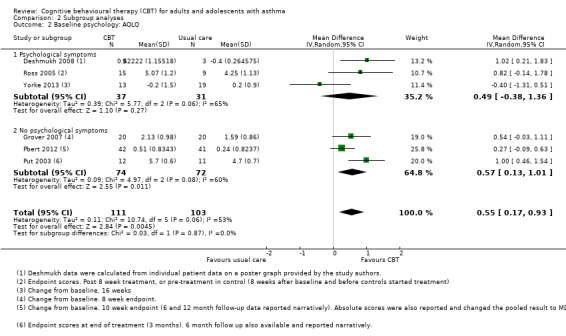
Comparison 2 Subgroup analyses, Outcome 2 Baseline psychology: AQLQ.
2.3. Analysis.
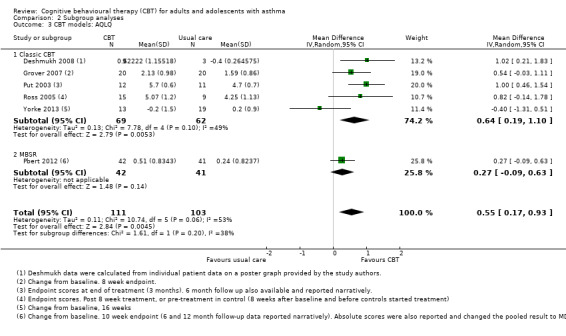
Comparison 2 Subgroup analyses, Outcome 3 CBT models: AQLQ.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Deshmukh 2008.
| Methods | Parallel RCT conducted in Australia. 5 weeks end of treatment with a 3‐month follow‐up | |
| Participants | 18 participants were randomised to CBT (10) or the control group (8) Baseline characteristics: Mean age was 46 (SD 12) in the CBT group and 53 (SD 13) in the control group. Percentage male was 40% in the CBT group and 12.5% in the control group Baseline psychological status: Inclusion criteria required participants to have anxiety but did not specify criteria Inclusion criteria: Participants identified with comorbid anxiety and asthma Exclusion criteria: Not reported |
|
| Interventions |
Intervention: 4‐session CBT intervention Delivered by: Qualifications not described Control: Asthma monitoring control group Amount of contact: Unclear duration of each session in the intervention group. The control group were given no additional contact. |
|
| Outcomes | Asthma‐related emotional functioning, AQLQ, HADS anxiety and depression subscales | |
| Notes | Funding: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details (conference abstract only). |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors could have been blind, but there was no description in the study of whether or how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 out of 8 people in the control group dropped out and were not included in the analysis (62.5%), compared with 1 out of 10 in the intervention group (10%). |
| Selective reporting (reporting bias) | High risk | Conference abstract only, no full publication. Emotional functioning and AQLQ were only reported dichotomously, and continuous scores were not available in the abstract. AQLQ scores were displayed graphically on the poster provided by the authors, which could be included in meta‐analysis, but not the HADS results. |
| Other bias | Low risk | None noted. |
Grover 2002.
| Methods | Parallel RCT conducted in 1 outpatient department in Bangalore, India. Duration of intervention was unclear as study was only available as a conference abstract. | |
| Participants | 10 participants were randomised to CBT (5) or the control group (5) No baseline characteristics reported as currently only available as a conference abstract Baseline psychological status: Not reported Inclusion criteria: No information Exclusion criteria: No information |
|
| Interventions |
Intervention: 15 individual sessions of CBT consisting of asthma education, Jacobson progressive muscle relaxation (JPMR), behavioural techniques, cognitive restructuring, cognitive coping skills, and behavioural counselling to significant others Delivered by: Qualifications not described Control: Standard pharmacotherapy alone Amount of contact: Unclear duration of each session in the intervention group or over how many weeks it was delivered. The control group were given no additional contact. |
|
| Outcomes | ASC, asthma diary, STAI, BDI, AQLQ, and PEFR | |
| Notes | Funding: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "experimental design with pre‐ and post‐therapy assessments" |
| Allocation concealment (selection bias) | High risk | "sequentially allotted to two groups", could have allowed for bias in allocation to groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors could have been blind, but there was no description in the study of whether or how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of any dropout ‐ minimal information in the conference abstract. |
| Selective reporting (reporting bias) | High risk | None of the outcomes were reported in sufficient detail to include in the meta‐analysis. |
| Other bias | Low risk | None noted. |
Grover 2007.
| Methods | Parallel RCT conducted in 1 outpatient department in Bangalore, India. The intervention lasted between 6 and 8 weeks, and data were collected over 23 months from November 1999 to October 2001 | |
| Participants | 40 participants were randomised to CBT plus self management (20) or self management alone (20) Baseline characteristics: Minimal reported ‐ no mean age, percent male, % smokers, or baseline lung function Baseline psychological status: Participants with a clinical history of psychiatric illness and those on anti‐anxiety and antidepressant medication were excluded Inclusion criteria: Individuals with a diagnosis of asthma (according to American Thoracic Society criteria 1987), age 18 to 45 years, duration of illness at least 2 years, and working knowledge of Hindi/English Exclusion criteria: People with other medical conditions involving breathing difficulties; presence of other medical conditions such as coronary heart disease, diabetes, or hypertension; clinical history of psychiatric illness; history of exposure to structured psychological intervention |
|
| Interventions |
Intervention: Asthma self management (as below) plus cognitive restructuring, skills training (problem solving, social), imaginary rehearsal, role‐plays, weekly activity schedule, and homework assignments Delivered by: "therapist" ‐ qualifications not described Control: ASMP based on National Institutes of Health criteria, modified to suit the population. Included asthma education, training in self management behaviour, guided self management plan, self management with an asthma diary, discussion on negative emotions and asthma, breathing exercises, and behavioural counselling to significant others Amount of contact: 15 one‐hour sessions in the intervention group and 10 one‐hour sessions in the control group. Both were given over 6 to 8 weeks. |
|
| Outcomes | SSIS, ASC, asthma diary, ABP, HADS, AQLQ, and PEFR | |
| Notes | Funding: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After informed consent and baseline assessment, patients were randomly allotted, using random number table" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors could have been blind, but there was no description in the study of whether or how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of any dropout. |
| Selective reporting (reporting bias) | Low risk | PEFR only available graphically, but the review did not consider lung function, and the SSIS was not reported, however again we did not consider this an important outcome for the review. |
| Other bias | High risk | "groups were comparable on socio‐demographic and clinical variables such as age, sex, marital status, education, religion, occupation, family history of asthma, work loss, hospital admission, duration of illness and emergency room visits. Groups were not comparable on baseline assessment on ABP‐B (P < 0.01), ASC (P < 0.05) and HADS (P < 0.01)." |
Parry 2012.
| Methods | Parallel RCT conducted at 16 medical centres in the UK. Investigators approached family doctors, outpatient and inpatient centres in Sheffield to identify participants. The intervention lasted between 6 and 13 weeks. | |
| Participants | 94 participants were randomised to CBT (50) or the control group (44) Baseline characteristics: Mean age was 44 in the CBT group and 43 in the control group. Percentage male was 32 in the CBT group and 39 in the control group. FEV not given but did state that 17/50 in the CBT group and 16/44 in the control group had severe asthma (> 25% reduction of FEV1). For both groups, baseline data were reported separately for those who completed and those who withdrew or were lost to follow‐up. We have merged the 2 groups to show the characteristics of all randomised participants in each group. Baseline psychological status: "highly anxious" as per HADS anxiety scale or ASC panic fear score cutoffs Inclusion criteria: Aged 18 to 65, clinical diagnosis of asthma, above threshold on clinical criteria of anxiety using published cutoff points on the HADS Anxiety and ASC panic fear subscale (ASC‐PF). Score of 8 or more on the HADS anxiety or 28 or more on the ASC‐PF were eligible. Asthma diagnosis was based on a clinical picture of airflow obstruction with diurnal variation in symptomatology and clinical evidence of airways hyper‐irritability. Exclusion criteria: HADS < 4, age under 18 or over 65, unable to read and complete questionnaire in English, severe psychiatric illness with history of hospital admission, diagnosed heart failure or angina, significant comorbid lung disease |
|
| Interventions |
Intervention: CBT with therapist based on asthma‐specific fears, promoting awareness of anxiety‐provoking cognitions and beliefs, controlled exposure and tolerance to reduce dysfunctional somatic preoccupation and safety‐seeking, breathing techniques, postural adjustments and relaxation for hyperventilation, identifying triggers to panic fear, and problem‐solving skills. The intervention group were followed up at 6 months. Delivered by: 4 therapists: 3 clinical psychologists and 1 cognitive behavioural therapist (none specialised in asthma) Control: Treatment delayed until the intervention post‐treatment measurement. The control group 'post‐treatment' assessment took place 3 months after baseline, and the follow‐up after 9 months Amount of contact: The intervention group had a 1.5‐hour introductory session followed by 4 to 6 sessions either weekly or fortnightly; treatment lasted between 6 and 13 weeks. The control group received no additional contact during the intervention phase. |
|
| Outcomes |
Primary clinical outcome measure: ASC‐PF at 6 months after end of treatment (clinically significant fear = 28). Secondary outcomes: EQ‐5D, HADS, ABP, AMHLC, all self‐completed at baseline, end of treatment, and 6 months after end of treatment (baseline, 3 months, and 9 months for control participants). Mean time to collection of the second endpoint data was 53 weeks for the treatment group (range 35 to 74 weeks) and 51 weeks for the control group (range 37 to 74 weeks). The ANCOVA analyses were adjusted for baseline ASC score, age, group, gender, and smoking. |
|
| Notes | Funding: Department of Health for England and Wales Asthma Management Programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "an independent statistician who was a member of data monitoring group generated a blocked and stratified by asthma severity and socioeconomic status randomisation schedule by computer" |
| Allocation concealment (selection bias) | Low risk | "The research associate assigned the participants to treatment groups in strict sequential order according to the schedule and informed them of the allocation by telephone" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors could have been blind, but there was no description in the study of whether or how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The main analyses were by intention to treat to reduce bias due to differential attrition from the intervention and control groups. For participants withdrawn on the basis of clinical assessment (Fig. 1) data at endpoint 1 and 2 were imputed as zero change as it was assumed that these participants would not have benefited from CBT. For all other missing data imputation was last observation carried forward." "All randomised patients were followed up and included in the analysis where data were available, irrespective of whether they completed treatment. More participants completed outcome measures at the second endpoint than at the first endpoint. The numbers analysed for each group were as follows: first endpoint: 20 CBT, 29 control; second endpoint: 28 CBT, 31 control." Data for only 20 of the 50 intervention group participants (40%) and 29 of the 44 control group participants (65%) were available at the end‐of‐treatment time point, representing very high and unbalanced dropout, which may not have been adequately controlled for by the imputation. |
| Selective reporting (reporting bias) | High risk | Results for the anxiety subscale of the HADS were only reported as "no significant difference". Number of participants admitted to hospital was only given for the period before treatment. |
| Other bias | Low risk | None noted. |
Pbert 2012.
| Methods | Parallel RCT conducted at a university hospital outpatient primary care and pulmonary care clinic in Massachusetts, USA. The intervention lasted for 8 weeks. | |
| Participants | 83 participants were randomised to MBSR (42) or the "Healthy Living Course" control group (41) Baseline characteristics: Mean age was 52 in the mindfulness group and 54 in the control group. Percentage male was 36% in the mindfulness group and 39% in the control group. Participants in the mindfulness group had a mean percentage predicted FEV1 of 91.7% (SD 16.6), and those in the control group had a mean of 94.6 (SD 18.9). 80.0% in the control group and 83.8% in the mindfulness group were on inhaled corticosteroid or oral prednisone. Many other baseline characteristics were also reported including race, education, marital status, asthma control category, asthma severity category, other lung function metrics, rescue inhaler and other medication use, AQLQ, PSS, school and work absence. Baseline psychological status: People with a psychiatric hospital admission in the previous 2 years or who had taken psychotropic medications in the past year were excluded. Inclusion criteria: Physician‐documented asthma with an objective indicator of bronchial hyper‐responsiveness (positive methacholine challenge test, at least 12% improvement in FEV1 or FVC in response to bronchodilator, or 20% variability in diurnal PEF variation), or at least 12% improvement in FEV1 in response to inhaled bronchodilator on spirometry at study entry (2007 NIH/NHLBI criteria for mild, moderate, or severe persistent asthma). Able to read and understand English, able to complete informed consent process and study data collection procedures Exclusion criteria: Intermittent asthma (symptoms less than once/week, brief exacerbations, nocturnal symptoms less than or equal to twice a month, and normal lung function between episodes); smoked in the past year; other lung diseases; current treatment for symptomatic cardiovascular disease; history of a positive tuberculosis test; participated in MBSR and/or practicing meditation regularly. Additional from NCT site: cancer except non‐melanoma skin cancer, on psychotropic medications in the prior 12 months, psychiatric hospitalisation in the past 2 years |
|
| Interventions |
Intervention: Participants were integrated into regularly scheduled MBSR classes, which had approximately 2 study and 28 non‐study participants. Mindfulness training included body scan, sitting meditation focusing on awareness of breathing, thoughts and feelings, and gentle stretching exercises to develop awareness during movement, emphasising integration into everyday life to support coping with symptoms and stress. 2 CDs containing guided mindfulness exercises were provided to be practiced for 30 minutes, 6 days/week Delivered by: Qualifications not described Control: HLC was offered to community members in addition to study participants and consisted of approximately 7 study and 18 non‐study participants. HLC matched the intervention for time, instructor attention, and format. Classes consisted of lectures and discussion of self care topics: healthy nutrition; physical activity; coping with stress (not including mindfulness); sleep hygiene; balancing work and personal life; and living a drug‐free life. Homework was assigned consistent in time with the MBSR group. Amount of contact: Participants in both groups received 8‐weekly 2.5‐hour sessions plus a 6‐hour session in week 6 |
|
| Outcomes | AQLQ change from baseline in 2‐week average morning PEFR, asthma control according the 2007 NIH/NHLBI guidelines, and PSS. At each assessment, participants recorded frequency of asthma rescue medication use (short‐acting bronchodilators) over a 14‐day period, and days of work or school missed due to asthma. Asthma exacerbations were assessed by self reported initiation of prednisone in the last 30 days. Follow‐up assessments were at 10 weeks, 6 months, and 12 months. |
|
| Notes | Funding: Grant R21 AT002938 (awarded to Drs Pbert and Carmody) from the NIH National Center for Complementary and Alternative Medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was by a random allocation scheme with block sizes of four and six". Suggests computerised schedule but unclear. |
| Allocation concealment (selection bias) | Low risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. The study made efforts to ensure the intervention and control were matched in many ways "to control for as many non‐specific factors as possible". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessments occurred at baseline, and at 10 weeks and 6 and 12 months post baseline by evaluators blind to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants followed up varied between 37 and 39 out of 42 in the mindfulness group at different time points (88% to 93%) and 37 to 41 out of 41 in the control group (90% to 100%), which represents low and balanced dropout. "For the peak flow/medication form and spirometry, there were up to 21 missing data points at follow‐up. For short‐term rescue medication use, 2‐week average morning PEF, PEF variability, and FEV1, missing values were extrapolated using the slope of the two closest non‐missing values; for 10 patients, single non‐missing values were carried forward to all subsequent time points. The results presented are from these imputed models." |
| Selective reporting (reporting bias) | Low risk | All of the named outcomes were well reported at all time points and were analysed as described in the prospectively registered protocol. |
| Other bias | Low risk | "Prednisone use differed between groups at baseline and was included in final models if associated with time trends and altered estimates of study arm effects." |
Put 2003.
| Methods | Parallel RCT conducted at a university hospital in Belgium. The intervention lasted for 6 sessions, and measurements were taken at 0, 3, and 6 months. | |
| Participants | 25 participants were randomised to CBT (13) or the control group (12) Baseline characteristics: Mean age was 43 (SD 10) in the CBT group and 48 (SD 12) in the control group. Percentage male was 58.3 in the CBT group and 36.4 in the control group. Participants in the CBT group had a mean percentage predicted FEV1 of 85 (SD 20); those in the control group had a mean of 90 (SD 12). Several other baseline characteristics were also reported including duration of symptoms, FEV1 (L), FVC (L) and %, prescribed medication, and severity of asthma. Baseline psychological status: No information Inclusion criteria: Diagnosed with asthma at least 6 months earlier Exclusion criteria: Age younger than 18 or older than 65 years, occupational asthma, nicotine, drug or alcohol abuse, absence of asthma symptoms during the last 6 months, brittle asthma, previous participation in an educational or other asthma programme |
|
| Interventions |
Intervention: Individual CBT: psycho‐education, behavioural techniques (self observation/monitoring, stimulus control, response control), cognitive restructuring including personalised elaboration on problem areas Delivered by: Trained psychologist Control: Waiting list Amount of contact: Participants in the control group received 6 one‐hour individual sessions. |
|
| Outcomes | McMaster AQLQ; ASC; Negative Emotionality Scale; Knowledge, Attitude, and Self‐Efficacy Asthma Questionnaire; Adherence Scale, and PEFR | |
| Notes | Funding: Fonds voor Wetenschappelijk Onderzoek‐Vlaanderen (Grant 7.0004.000) and Astra Pharmaceuticals, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated to either a programme group or a waiting list control group by means of the envelope technique" |
| Allocation concealment (selection bias) | Low risk | "This randomisation method consists of drawing for each subject one unmarked, non‐transparent envelope from a total of 23 envelopes (i.e. number of participants; 12 for treatment and 11 for control condition) containing the name of either condition." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | '"Two independent researchers were responsible for conducting the programme and for performing the measures. The person who collected the data was unaware of the condition each participant was assigned to." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "From the treatment group, one subject dropped out after onset of the programme, (organisational incompatibility with professional situation), and from the waiting list group, not all the data were collected for one subject. Eventually, 23 subjects were included in the study: 12 subjects in the treatment group, and 11 subjects in the control group." Data were not imputed for non‐completers, but unlikely to bias results since it was only 1 participant per group. |
| Selective reporting (reporting bias) | Low risk | All of the named outcomes were well reported at all time points and were analysed as described, although there was no prospectively registered protocol to check. |
| Other bias | Low risk | "The control group was prescribed more anticholinergics than the intervention group (Chi‐squared = 5.3, P = 0.02); both conditions did not differ regarding other characteristics. Asthma severity was equal for both groups, only one subject was categorised as severely asthmatic." |
Ross 2005.
| Methods | Parallel RCT conducted in Canada. "Participants in the treatment condition were assessed on three occasions: pretreatment, posttreatment, and 6‐month follow‐up. Participants in the wait‐list condition were assessed on four occasions: baseline (which coincided with the experimental condition pretreatment), pretreatment (which coincided with the experimental condition posttreatment assessment), posttreatment, and 6‐month follow‐up." | |
| Participants | 48 participants were randomised to CBT (25) or the control group (23) (although only 15 and 10, respectively were included in the analysis) Baseline characteristics: Mean age was 37.9 (SD 10.5) in the CBT group and 40.7 (SD 12.6) in the control group. All participants were female. Mean percentage predicted FEV1 was 76 (18) pre‐ and 94 (5) post‐bronchodilator for participants in the CBT group and 81 (16) pre‐ and 95 (4) post‐bronchodilator for those in the control group. Baseline psychological status: Primary diagnosis of panic disorder (determined by severity) with no, mild, or moderate agoraphobic avoidance, at least 3 panic attacks in the past 3 weeks Inclusion criteria: Physician‐assigned diagnosis of asthma and who had been referred to a pulmonary specialist or who had recently sought ED care for an acute asthma episode, a primary diagnosis of panic disorder (determined by severity) with no, mild, or moderate agoraphobic avoidance, at least 3 panic attacks in the past 3 weeks Exclusion criteria: Recent change in psychotropic medication type or dose, medical condition that would contraindicate protocol participation or that would confuse the interpretation of the results, for example emphysema, organic brain syndrome, bipolar disorder, schizophrenia, obsessive‐compulsive disorder, and alcohol or drug dependence |
|
| Interventions |
Intervention: Derived from Barlow panic control treatment and Beck cognitive treatment for panic disorder. The CBT portion included education about the nature, etiology, and maintenance of anxiety and panic, cognitive therapy techniques, training in slow diaphragmatic breathing, and interoceptive exposure exercises. The asthma education program consisted of information about airways inflammation and bronchospasm, rescue and controller medication, methods of self monitoring, triggers, action plans, reviewing asthma diaries, and the overlap/interplay of asthma and panic. Delivered by 2 nurse clinicians in small groups of 3 to 5 participants. Delivered by: 2 doctorally prepared nurse clinicians, 1 trained as an asthma educator and 1 with postdoctoral training in CBT Control: No treatment. Participants were offered the intervention after the study had finished. Amount of contact: Participants in the treatment group received 12 90‐minute sessions over 8 weeks. Sessions 1 through 8 were conducted twice weekly, and sessions 9 through 12 were spaced 1 week apart. |
|
| Outcomes | Panic attack diary, SPRAS, ASI, FQ‐Ago, BDI, asthma symptom‐free days, morning PEFR, and peak‐flow variability from Asthma Symptom Diaries, AQLQ | |
| Notes |
Funding: Funded in part by the Alberta Heritage Foundation for Medical Research, the Alberta Lung Association, and the Canadian Lung Association. Due to the design of the study, we extracted data at post‐treatment for the experimental group and at pre‐treatment for the control group (i.e. after randomisation but before they were also given the intervention). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Describes in detail how participants were screened over the phone but not the schedule for randomisation, just "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Does not state who actually assigned the participants to groups and how |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors could have been blind, but there was no description in the study of whether or how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "48 participants were offered and accepted a place in the CBT‐AE program and were randomly assigned to either the experimental treatment condition (n = 24) or the wait‐list condition (n = 24). Fourteen of these participants (11 in the wait‐list condition) dropped out prior to treatment, leaving 34 who commenced treatment. Nine of these 34 participants withdrew from treatment for a variety of reasons unrelated to treatment." Outcomes and demographic characteristics are reported for the remaining 25 (15 experimental, 10 control) participants and not for the whole sample. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in appropriate detail. |
| Other bias | Low risk | "When these pretreatment dropouts (n = 14) were compared with the treatment completers (n = 25), using t‐tests or chi squares where appropriate, no significant between‐group differences were found on any of the demographic variables (age, marital status, education, income) or any of the clinical variables (self‐rated asthma severity, years since asthma diagnosis, average number of asthma medications, average number of comorbid anxiety disorders, proportion on psychotropic medications, or other medications). Moreover, separate analyses revealed no significant between‐group differences on the SPRAS, FQ‐Ago, ASI, BDI, and AQLQ scores obtained at baseline (in the case of participants assigned to the wait‐list condition) or pretreatment (in the case of participants assigned to the experimental treatment condition)." |
Sommaruga 1995.
| Methods | Parallel RCT conducted at a medical centre in Italy. Measurements were taken at baseline while participants were admitted to hospital and a year later | |
| Participants | 40 participants were randomised to CBT (20) or the control group (20) Baseline characteristics: Mean age was 44 (SD 16) in the CBT group and 51 (SD 16) in the control group. Percentage male was 55 in the CBT group and 45 in the control group. Mean percentage predicted FEV1 was 76 (SD 18) pre‐bronchodilator and 94 (SD 5) post‐bronchodilator for the CBT group, and 81 (SD 16) pre‐bronchodilator and 95 (SD 4) post‐bronchodilator for the control group. Several other baseline characteristics were also reported, including mean duration of asthma diagnosis. Baseline psychological status: No information Inclusion criteria: Asthma diagnosed, treated, and followed up according to 1987 American Thoracic Society guidelines Exclusion criteria: Not well described |
|
| Interventions |
Intervention: ARG: Educational programme consisting of meetings (twice in hospital and quarterly throughout the following year) with physician, physiotherapist and psychologist, daily peak flow meter, telephone access to physician, personal medication plan, followed up 6 times a year by the physician. CBT intervention was given during 3 individual meetings with the psychologist covering cognitive restructuring, education on symptoms and emotional reactions to them, behaviour modification, use of drugs and psychological aspects of anxiety, relaxation training. Delivered by: Trained psychologist Control: The control group did not receive an educational programme or psychological intervention. They were treated according to NHLBI 1991 guidelines and followed up 6 times/year by the physician with examination and spirometry. Amount of contact: 6 educational sessions (2 in hospital and 4 out of hospital) + 3 sessions of CBT + 6 physician visits. The control just received 6 physician visits. |
|
| Outcomes | STAI, QD, QPF (not defined but described as assessing psychophysiological disorders, as part of the Cognitive Behavioural Assessment), ASC in Italian to assess the emotional reactions to asthmatic crises (i.e. panic‐fear), Respiratory Illness Opinion Survey in Italian, Health Locus of Control Scale in Italian, plus clinical interview. All at baseline and 1 year later. | |
| Notes | Funding: No information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly assigned to an Asthma Rehabilitation Group…or a Control Group." |
| Allocation concealment (selection bias) | Unclear risk | '"Forty consecutive patients were enrolled"; no other details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors could have been blind, but there was no description in the study of whether or how this was done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | '"No patients from the ARG dropped out of the study, whilst four (20%) of the CG dropped out during the follow‐up" |
| Selective reporting (reporting bias) | High risk | Key clinical outcomes only reported in detail at baseline (attacks, hospitalisation days, emergency visits, and work/school absences). Also, ASC "not considered because seven patients from the AR had no further asthmatic crises in the period following enrolment, thus making the compilation of the test at follow‐up, and statistical comparison, impossible". |
| Other bias | High risk | The intervention group received additional interventions, which may have confounded the result. |
Yorke 2013.
| Methods | Parallel RCT conducted at 2 tertiary hospitals in England. The intervention lasted for 8 weeks, and measurements were taken at 0, 8, and 16 weeks. | |
| Participants | 51 participants were randomised to CBT (25) or the control group (26) Baseline characteristics: Mean age was 48.6 (SD 11.1) in the CBT group and 45.0 (SD 13.7) in the control group. Percentage male was 35 in the CBT group and 52 in the control group. Several other baseline characteristics were also reported including ethnicity, previous counselling, HADS, AQLQ, ACQ, D12, and EuroQol baseline scores Baseline psychological status: HADS score > 8 for either subscale Inclusion criteria: Participants from 2 tertiary hospitals in England attending 1 of a small subgroup of national specialist severe asthma clinics were screened for the following eligibility criteria: adults (≥ 18 years of age) with a confirmed diagnosis of severe refractory asthma (ATS 2000) and receiving standard‐of‐care therapy at BTS Steps 4 and 5 level. Participants were routinely screened for the presence of clinically significant anxiety or depression, or both using the HADS (score > 8 for anxiety or > 8 for depression). Exclusion criteria: People with a specific psychiatric condition (e.g. schizophrenia, hypomania) |
|
| Interventions |
Intervention: Manual‐guided group CBT (Antoni) with focus on relaxed breathing for anxiety‐related breathlessness and personal goal‐setting, with a CD to help participants practice relaxation between sessions. Topics covered included stress and awareness of asthma exacerbations, linking thoughts and emotions, cognitive distortions, building resilience/coping strategies, problem‐solving, communication, and social support Delivered by: Trained clinical psychology therapists Control: Usual care only Amount of contact: Participants in the CBT group received 8 1.5‐hour weekly sessions. Control group participants did not receive any additional contact. |
|
| Outcomes | AQLQ, ACQ, HADS, asthma diary, acceptability, D12, EQ‐5D, and EQ‐VAS | |
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out independently by the Clinical Trials and Evaluation Unit (CTEU), Royal Brompton and Harefield National Health Service Foundation Trust (RBHT). |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out independently by the Clinical Trials and Evaluation Unit (CTEU), Royal Brompton and Harefield National Health Service Foundation Trust (RBHT). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions could not be kept blind from participants and personnel. By default, this is high risk of bias for subjective outcomes including self rated questionnaires, but probably would not introduce bias for more objective outcomes such as exacerbations and adverse events. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection‐design facilitated research nurse (RN) blinding to group allocation, however this was difficult to maintain as participants often discussed their treatment with the RN at subsequent study follow‐ups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | '"Seven subjects (3 G‐CBT and 4 control) who undertook the study were later found not to meet the inclusion criteria, and were removed from all analyses, leaving 44 (from 51 randomised). Participants allocated to receive G‐CBT but withdrew prior to starting treatment (n = 3) or provided baseline data only (n = 2; attended 2 or less sessions) were removed from further analyses. For each variable there was less than 10% missing data. The only exception was asthma diaries (discussed below)." Numbers in the outcome table are 13 and 18‐19. |
| Selective reporting (reporting bias) | Low risk | All of the named outcomes were well reported at all time points and were analysed as described in the published reports or via the study author (JY). |
| Other bias | Low risk | None noted. |
ABP = Asthma Bother Profile
ACQ = Asthma Control Questionnaire
AMHLC = Asthma Multidimensional Health Locus of Control
ANCOVA = analysis of covariance
AQLQ = Asthma Quality of Life Questionnaire
ARG = Asthma Rehabilitation Group
ASC‐PF = Asthma Symptom Checklist Panic‐Fear subscale
ASI = Anxiety Sensitivity Index
ASMP = asthma self management program
BDI = Beck Depression Inventory
BTS = British Thoracic Society
CBT = cognitive behavioural therapy
D12 = Dyspnoea‐12 questionnaire
ED = emergency department
EQ‐5D = EuroQol 5D
EQ‐VAS = EuroQol visual analogue scale
FEV1 = forced expiratory volume in one second
FQ‐Ago = Fear Questionnaire ‐ Agoraphobia subscale
FVC = forced vital capacity
HADS = Hospital Anxiety and Depression Scale
HLC = Healthy Living Course
MBSR = Mindfulness‐Based Stress Reduction
NCT = National Clinical Trials (clinicaltrials.gov)
NIH/NHLBI = National Institutes of Health/National Heart, Lung, and Blood Institute
PEFR = peak expiratory flow rate
PSS = Perceived Stress Scale
RCT = randomised controlled trial
SD = standard deviation
SPRAS = Sheehan Patient‐Rated Anxiety Scale
SSIS = semi‐structured interview schedule
STAI = State‐Trait Anxiety Inventory
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12613000675729 | Wrong population ‐ children |
| Bailey 1987 | Wrong design ‐ not an RCT. Not CBT |
| Balfour 1957 | Wrong design ‐ not an RCT |
| Baptist 2013 | Wrong intervention ‐ self management |
| Barendregt 1957 | Wrong design ‐ not an RCT |
| Benedito 1996 | Wrong intervention and comparison ‐ 3 non‐CBT therapies and no usual care comparator |
| Bosley 1995 | Wrong design ‐ allocation not random |
| Charlson 2007 | Wrong intervention and mixed population ‐ purely behavioural |
| Chen 2010 | Wrong intervention ‐ self efficacy |
| ChiCTR‐COC‐15007442 | Wrong design ‐ case‐control study |
| Clark 2004 | Wrong intervention ‐ self management |
| Deenen 1996 | Wrong population ‐ severe asthma and COPD. Data for those with asthma not available separately. |
| Deter 1983 | Wrong intervention ‐ purely relaxation therapy rather than full CBT |
| Epstein 2004 | Wrong intervention ‐ mental imagery |
| Hampel 2003 | Wrong population ‐ children and adolescents with a mean age of 11.6 |
| Hock 1978 | Wrong population and intervention ‐ children and not CBT |
| Holloway 2007 | Wrong intervention ‐ Papworth breathing techniques |
| Jerant 2008 | Wrong intervention and mixed population ‐ not testing CBT |
| Khoshnavay 2013 | Wrong population ‐ children |
| Kotses 1995a | Wrong intervention ‐ self management |
| Lewandowska 2006 | Wrong design ‐ not randomly allocated |
| Mancuso 2010 | Wrong intervention ‐ self management education |
| Mancuso 2012 | Wrong intervention ‐ self efficacy not CBT |
| Miklich 1977 | Wrong design ‐ not randomly allocated |
| Mildenhall 1997 | Wrong intervention ‐ coping skills program |
| Milenković 2007 | Wrong intervention ‐ self management program |
| Moore 1965 | Wrong design and intervention ‐ within‐patient comparison and solely behavioural intervention |
| Perrin 1992 | Wrong population ‐ children |
| Philipp 1972 | Wrong design ‐ not an RCT |
| Sanger 1969 | Wrong intervention and design ‐ not a CBT intervention and unlikely to be an RCT |
| Smith 2005 | Wrong intervention ‐ psycho‐education |
| Smith 2015 | Wrong intervention ‐ written emotional disclosure |
| Song 2005 | Wrong intervention ‐ mostly relaxation, and not properly randomised |
| Spiess 1988 | Wrong intervention ‐ "information and relaxation groups" |
| Srof 2012 | Wrong intervention ‐ self efficacy |
| Stone 2000 | Wrong intervention ‐ written emotional disclosure |
| Theadom 2010 | Wrong intervention ‐ written emotional disclosure |
| Tong 2002 | Wrong population ‐ children |
| van Gaalen 2013 | Wrong intervention ‐ internet‐based management support |
| Vazquez 1993 | Wrong intervention ‐ relaxation therapy |
| Vazquez 1993a | Wrong intervention ‐ relaxation therapy |
| Wilkening 1999 | Wrong intervention ‐ only behavioural elements |
CBT = cognitive behavioural therapy
COPD = chronic obstructive pulmonary disease
RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000915651.
| Trial name or title | Randomised controlled trial of telephone based CBT for patients with chronic lung disease and anxiety and/or depression undergoing pulmonary rehabilitation to evaluate the effect on symptoms of anxiety and depression, quality of life, and exacerbations |
| Methods | Parallel randomised control trial |
| Participants | Mixed population with chronic lung disease ‐ may not meet inclusion criteria |
| Interventions | Intervention will be 6 CBT sessions administered by psychology interns. The 6 sessions will include 2 individual face‐to‐face sessions (an hour each, within the first 4 weeks of pulmonary rehabilitation) and 4 phone sessions (an hour each, fortnightly within the first 2 months after the face‐to‐face sessions). The comparator will be usual care comprised of medical treatment and pulmonary rehabilitation. |
| Outcomes | Primary outcomes: Symptoms of anxiety using GAI; symptoms of depression using GDS Secondary outcomes: 6MWD; SGRQ; asthma patients will also answer the AQLQ and ACQ; emergent healthcare utilisation (primary care and hospital care) assessed by data linkage to patient medical records and a questionnaire (designed for this study to assess exacerbation rate) at 6‐ and 12‐month intervals. Pulmonary rehabilitation attendance and a structured interview aimed to assess participation. |
| Starting date | Not yet recruiting |
| Contact information | Professor Ian Yang and Dr Marsus I Pumar, both at The Prince Charles Hospital, Queensland Australia |
| Notes | www.anzctr.org.au/ACTRN12614000915651.aspx apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12614000915651 |
IRCT2015061622770N1.
| Trial name or title | The impact of mindfulness‐based cognitive therapy on anxiety and depression and somatic symptoms in patients with asthma |
| Methods | Randomly allocated to intervention and control groups using sealed envelopes. Not blind. Parallel. "This project is an empirical study of pre‐ and post‐test." Sample size 30 Random participants in the control group or the experiment will be replaced. |
| Participants |
Inclusion criteria: Women aged 55 to 18, at least 1 year since asthma diagnosis, high school education or above, ongoing medical treatment Exclusion criteria: Risk for psychotic disorder or other physical illness and absenteeism on more than 2 treatment sessions |
| Interventions |
Interventions: Mindfulness‐based cognitive therapy, 2‐hour sessions, 8 sessions per week Control group: Placed on a waiting list and will not receive any intervention. |
| Outcomes | The instruments included Beck Depression Inventory, the Beck Anxiety Inventory, and AQLQ. Measured before and immediately after the intervention. |
| Starting date | April 2015 ‐ retrospective registration |
| Contact information | Dr Ramani Ghasemi, Asthma Clinic, Jesus son of Mary Hospital, Esfahan, Iran |
| Notes | apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015061622770N1 |
NCT01583296.
| Trial name or title | Adaptation of a behavioral treatment for Latinos with panic disorder and asthma |
| Methods | Parallel, double‐blind randomised controlled trial |
| Participants | Enrolment 53 |
| Interventions |
Intervention: CBT and heart rate variability biofeedback Control group: Music Relaxation Therapy (may not meet the review inclusion criteria as not usual care) |
| Outcomes |
Primary: Panic disorder severity scale and use of quick‐relief medication for asthma Secondary: ACQ, Clinical Global Impression Scale, adherence with controller medications for asthma All measured as change from pre‐intervention to post‐intervention (8 weeks). |
| Starting date | July 2010 |
| Contact information | Jonathan Feldman, Albert Einstein College of Medicine of Yeshiva University |
| Notes | clinicaltrials.gov/ct2/show/NCT01583296 |
6MWD = 6‐minute walk distance
ACQ = Asthma Control Questionnaire
AQLQ = Asthma Quality of Life Questionnaire
CBT = cognitive behavioural therapy
GAI = Geriatric Anxiety Inventory
GDS = Geriatric Depression scale
SGRG = St George's Respiratory Questionnaire
Differences between protocol and review
Valdeep Dulay joined the author team after the protocol was published and contributed to the screening of abstracts, 'Risk of bias' judgements, and data extraction along with KK and MN as planned, so his initials have been added.
We had planned for one review author (KK) to extract study characteristics, but this was also done by a second review author (MN or VD) in order to reduce bias and potential for error.
We were unable to pool more than 10 studies, and so could not create and examine a funnel plot to explore possible small‐study and publication biases as planned in the protocol.
We planned to conduct subgroup analyses on the three primary outcomes: asthma‐related quality of life, exacerbations requiring at least a course of oral steroids, and asthma control. While we did not specify a minimum number of studies to conduct the subgroup analyses, only three studies contributed data to the second and third primary outcomes, which we did not consider to be sufficient for subgroup analyses. As such, we conducted subgroup analyses on the asthma‐related quality of life outcome only.
We added a justification for the two primary outcomes on the recommendation of a peer referee, and explained the reasoning behind the omission of an 'adverse events' outcome. We also added more detail to the inclusion criteria relating to the control groups (usual care or a minimal‐intervention control group) due to uncertainty that arose when deciding whether to include or exclude studies. We removed the comparator 'versus usual care' from the title due to variation in the control groups among studies (no treatment, waiting list, etc.).
Contributions of authors
KK: background and methods, sifting search results, data extraction, risk of bias, data analysis, GRADE, results write‐up, discussion.
MN: input in background and methods, sifting search results and inclusion/exclusion decisions, data extraction, risk of bias, discussion.
VD: sifting search results and inclusion/exclusion decisions, data extraction, risk of bias, abstract and plain language summary.
JY: inclusion/exclusion decisions, GRADE checking, discussion.
Sources of support
Internal sources
-
Kayleigh Kew, UK.
Supported by St George's, University of London
External sources
-
National Institute for Health Research, UK.
Evidence to guide care in adults and children with asthma, 13/89/14
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
Kayleigh Kew: none known.
Marina Nashed: none known.
Valdeep Dulay: none known.
Janelle Yorke is the primary author of one of the included studies. Data extraction and 'Risk of bias' judgements were completed by the other review authors.
New
References
References to studies included in this review
Deshmukh 2008 {published data only}
- Deshmukh V, Taylor W. Cognitive‐behavioral interventions targeting improved adult asthma control and quality of life. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12605000374662 (accessed 5 February 2016).
- Deshmukh V, Toelle B, Usherwood T, O'Grady B, Jenkins C. Evaluation of psychological intervention in adults with anxiety and asthma targeting asthma‐related quality of life [Abstract]. Respirology 2008;13(Suppl s2):A40. [DOI] [PubMed] [Google Scholar]
- Deshmukh VM, Toelle BG, Usherwood T, O'Grady FB, Jenkins CR. Evaluation of a psychological intervention in adults with anxiety and asthma targeting asthma‐related quality of life [Abstract]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2008:Poster #C88.
Grover 2002 {published data only}
- Grover N, Kumaraiah V, Prasadrao PS, D'Souza G. Cognitive behavioural intervention in bronchial asthma. Journal of the Association of Physicians of India 2002;50:896‐900. [PubMed] [Google Scholar]
Grover 2007 {published data only}
- Grover N, Souza G, Thennarasu K, Kumaraiah V. Randomized controlled study of CBT in bronchial asthma. Lung India 2007;24(2):45‐50. [Google Scholar]
Parry 2012 {published data only}
- Cooper C. Cognitive behaviour therapy in improving self‐management of asthma‐related anxiety. 34th International Meeting of the Society for Psychotherapy Research; 2003 June 25‐29; Weimar. 2003:144.
- Parry G. Clinical and cost‐effectiveness of a cognitive behavioural intervention for improved self‐management in adults with psychological complications of asthma. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN45927583 (accessed 5 February 2016).
- Parry GD, Cooper CL, Moore JM, Yadegarfar G, Campbell MJ, Esmonde L, et al. Cognitive behavioural intervention for adults with anxiety complications of asthma: prospective randomised trial. Respiratory Medicine 2012;106(6):802‐10. [DOI] [PubMed] [Google Scholar]
Pbert 2012 {published data only}
- Pbert L, Madison JM, Druker S, Olendzki N, Magner R, Reed G, et al. Effect of mindfulness training on asthma quality of life and lung function: a randomised controlled trial. Thorax 2012;67(9):769‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Put 2003 {published data only}
- Put C, Bergh O, Lemaigre V, Demedts M, Verleden G. Evaluation of an individualised asthma programme directed at behavioural change. European Respiratory Journal 2003;21:109‐15. [DOI] [PubMed] [Google Scholar]
Ross 2005 {published data only}
- Ross C, Davis T, MacDonald GF. The impact of a combined cognitive‐behavioural treatment and asthma education program for women with asthma and co‐existing panic disorder. American Journal of Respiratory and Critical Care Medicine 2002;165(8 (Suppl 2)):A434. [Google Scholar]
- Ross CJ, Davis TM, MacDonald GF. Cognitive‐behavioral treatment combined with asthma education for adults with asthma and coexisting panic disorder. Clinical Nursing Research 2005;14(2):131‐57. [DOI] [PubMed] [Google Scholar]
Sommaruga 1995 {published data only}
- Sommaruga M, Spanevello A, Migliori GB, Neri M, Callegari S, Manjani G. The effects of a cognitive behavioural intervention in asthmatic patients. Monaldi Archives for Chest Disease 1995;50:398‐402. [PubMed] [Google Scholar]
Yorke 2013 {published data only}
- Yorke J, Adair P, Doyle A‐M, Dubrow‐Marshall L, Fleming S, Holmes L, et al. A randomised controlled feasibility trial of group cognitive behavioural therapy for people with severe asthma. Journal of Asthma (in press). [DOI] [PubMed]
- Yorke J, Fleming S, Shuldham C, Doyle AM, Menzies‐Gow A, Adair P, et al. Feasibility and acceptability of group‐CBT for people with severe asthma [Abstract]. European Respiratory Journal 2013;42(Suppl 57):12s [213]. [Google Scholar]
References to studies excluded from this review
ACTRN12613000675729 {unpublished data only}
- Sharpe L, Dudeney J. A randomized trial of the efficacy of cognitive behavioural therapy for children with asthma and their parents. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613000675729 (accessed 5 February 2016).
Bailey 1987 {published data only}
- Bailey WC, Richards JM Jr, Manzella BA, Windsor RA, Brooks CM, Soong SJ. Promoting self‐management in adults with asthma: an overview of the UAB program. Health Education Quarterly 1987;14:345‐55. [DOI] [PubMed] [Google Scholar]
Balfour 1957 {published data only}
- Balfour Sclare A, Crocket JA. Group psychotherapy in bronchial asthma. Journal of Psychosomatic Research 1957;2:157‐71. [DOI] [PubMed] [Google Scholar]
Baptist 2013 {published data only}
- Baptist AP, Ross JA, Yang Y, Song PX, Clark NM. A randomized controlled trial of a self‐regulation intervention for older adults with asthma. Journal of the American Geriatrics Society 2013;61:747‐53. [DOI] [PubMed] [Google Scholar]
Barendregt 1957 {published data only}
- Barendregt JT. A psychological investigation of the effect of group psychotherapy in patients with bronchial asthma. Journal of Psychosomatic Research 1957;2:115‐9. [DOI] [PubMed] [Google Scholar]
Benedito 1996 {published data only}
- Benedito MC, Botella C, López JA. Influence of three psychological treatments on personality dimensions in asthmatic children. Anales de Psicología 1996;12:217‐22. [Google Scholar]
Bosley 1995 {published data only}
- Bosley CM, Fosbury JA, Cochrane GM. Psychological intervention to improve patient self‐management in asthma: the use of cognitive‐analytic therapy. European Respiratory Journal 1995;8:317S. [PubMed] [Google Scholar]
Charlson 2007 {published data only}
- Charlson ME, Boutin‐Foster C, Mancuso CA, Peterson JC, Ogedegbe G, Briggs WM, et al. Randomized controlled trials of positive affect and self‐affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: rationale, trial design, and methods. Contemporary Clinical Trials 2007;28:748‐62. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen SY, Sheu S, Chang CS, Wang TH, Huang MS. The effects of the self‐efficacy method on adult asthmatic patient self‐care behavior. Journal of Nursing Research 2010;18:266‐74. [DOI] [PubMed] [Google Scholar]
ChiCTR‐COC‐15007442 {published data only}
- Yuan Y, Zhang Y. An investigation of therapeutic mechanism in asthmatic patients: based on the results of Group Cognitive Behavioral Therapy. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐COC‐15007442 (accessed 5 February 2016).
Clark 2004 {published data only}
Deenen 1996 {published data only}
- Deenen T, Weerdt I, Jonkers R, Klip EC. Increasing the self‐management behavior of severe asthma and COPD patients by cognitive behavioural treatment. European Respiratory Journal 1996;9:305s. [Google Scholar]
Deter 1983 {published data only}
- Deter HC, Allert G. Group therapy for asthma patients: a concept for the psychosomatic treatment of patients in a medical clinic ‐ a controlled study. Psychotherapy and Psychosomatics 1983;40:95‐105. [DOI] [PubMed] [Google Scholar]
Epstein 2004 {published data only}
- Epstein GN, Halper JP, Barrett EA, Birdsall C, McGee M, Baron KP, et al. A pilot study of mind‐body changes in adults with asthma who practice mental imagery. Alternative Therapies in Health and Medicine 2004;10:66‐71. [PubMed] [Google Scholar]
Hampel 2003 {published data only}
- Hampel P, Rudolph H, Stachow R, Petermann F. Multimodal patient education program with stress management for childhood and adolescent asthma. Patient Education and Counseling 2003;49:59‐66. [DOI] [PubMed] [Google Scholar]
Hock 1978 {published data only}
- Hock RA, Rodgers CH, Reddi C, Kennard DW. Medico‐psychological interventions in male asthmatic children: an evaluation of physiological change. Psychosomatic Medicine 1978;40:210‐5. [DOI] [PubMed] [Google Scholar]
Holloway 2007 {published data only}
- Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax 2007;62:1039‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerant 2008 {published data only}
- Jerant A, Moore M, Lorig K, Franks P. Perceived control moderated the self‐efficacy‐enhancing effects of a chronic illness self‐management intervention. Chronic Illness 2008;4:173‐82. [DOI] [PubMed] [Google Scholar]
Khoshnavay 2013 {published data only}
- Khoshnavay Fomani F, Kharazmi Rahim Abadi R. The effectiveness of rational emotive behavior therapy (REBT) on the quality of life in asthmatic children. Iranian Journal of Allergy, Asthma, and Immunology 2013;12(9):S130. [Google Scholar]
Kotses 1995a {published data only}
- Kotses H, Bernstein IL, Bernstein DI, Reynolds RV, Korbee L, Wigal JK, et al. A self‐management program for adult asthma. Part I: Development and evaluation. Journal of Allergy and Clinical Immunology 1995;95:529‐40. [DOI] [PubMed] [Google Scholar]
Lewandowska 2006 {published data only}
- Lewandowska K, Rogiewicz M, Specjalski K, Niedoszytko M, Jassem E. The influence of psychotherapy on the quality of life in patients with moderate and severe bronchial asthma ‐ preliminary report. Polska Medycyna Paliatywna 2006;5:114‐9. [Google Scholar]
Mancuso 2010 {published data only}
- Mancuso CA, Sayles W, Allegrante JP. Randomized trial of self‐management education in asthmatic patients and effects of depressive symptoms. Annals of Allergy, Asthma & Immunology 2010;105:12‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancuso 2012 {published data only}
- Mancuso A, Choi TN, Westermann H, Wenderoth S, Hollenberg JP, Wells MT, et al. Increasing physical activity in patients with asthma through positive affect and self‐affirmation: a randomized trial. Archives of Internal Medicine 2012;172:337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miklich 1977 {published data only}
- Miklich DR, Renne CM, Creer TL, Alexander AB, Chai H, Davis MH, et al. The clinical utility of behavior therapy as an adjunctive treatment for asthma. Journal of Allergy and Clinical Immunology 1977;60:285‐94. [DOI] [PubMed] [Google Scholar]
Mildenhall 1997 {published data only}
- Mildenhall S, McLean M, Pearce S, Noble M, Harrison BDW. Evaluation of a home based coping skills training programme for high risk asthma sufferers. Thorax 1997;52:A46 P62. [Google Scholar]
Milenković 2007 {published data only}
- Milenković B, Bosnjak‐Petroić V. Self‐management program in treatment of asthma. Srpski Arhiv za Celokupno Lekarstvo 2007;135:147‐52. [DOI] [PubMed] [Google Scholar]
Moore 1965 {published data only}
- Moore N. Behavioral therapy in bronchial asthma: a controlled study. Journal of Psychosomatic Research 1965;9:257‐76. [DOI] [PubMed] [Google Scholar]
Perrin 1992 {published data only}
- Perrin JM, MacLean WE Jr, Gortmaker SL, Asher KN. Improving the psychological status of children with asthma: a randomized controlled trial. Journal of Developmental and Behavioral Pediatrics 1992;13:241‐7. [PubMed] [Google Scholar]
Philipp 1972 {published data only}
- Philipp RL, Wilde GJ, Day JH. Suggestion and relaxation in asthmatics. Journal of Psychosomatic Research 1972;16:193‐204. [DOI] [PubMed] [Google Scholar]
Sanger 1969 {published data only}
- Sanger MD. The treatment of anxiety and depression in the allergic patient. Annals of Allergy 1969;27:506‐10. [PubMed] [Google Scholar]
Smith 2005 {published data only}
- Smith JR, Mildenhall S, Noble MJ, Shepstone L, Koutantji M, Mugford M, et al. The Coping with Asthma Study: a randomised controlled trial of a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax 2005;60:1003‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2015 {published data only}
- Smith HE, Jones CJ, Hankins M, Field A, Theadom A, Bowskill R, et al. The effects of expressive writing on lung function, quality of life, medication use, and symptoms in adults with asthma: a randomized controlled trial. Psychosomatic Medicine 2015;77:429‐37. [DOI] [PubMed] [Google Scholar]
Song 2005 {published data only}
- Song X, Lu L, Li D. Influence of psychological intervention on emotional state of asthma patients. Chinese Nursing Research 2005;19:2008‐11. [Google Scholar]
Spiess 1988 {published data only}
- Spiess K, Sachs G, Buchinger C, Röggla G, Schnack C, Haber P. The effect of information and relaxation groups on lung function and the psychophysical status of asthma patients. Praxis und Klinik der Pneumologie 1988;42:641‐4. [PubMed] [Google Scholar]
Srof 2012 {published data only}
- Srof BJ, Velsor‐Friedrich B, Penckofer S. The effects of coping skills training among teens with asthma. Western Journal of Nursing Research 2012;34:1043‐61. [DOI] [PubMed] [Google Scholar]
Stone 2000 {published data only}
- Stone AA, Smyth JM, Kaell A, Hurewitz A. Structured writing about stressful events: exploring potential psychological mediators of positive health effects. Health Psychology 2000;19:619‐24. [PubMed] [Google Scholar]
Theadom 2010 {published data only}
- Theadom A, Smith H, Horne R, Bowskill R, Apfelbacher CJ, Frew AJ. Participant experiences of a written emotional disclosure intervention in asthma. Stress and Health 2010;26:45‐50. [Google Scholar]
Tong 2002 {published data only}
- Tong R, Pan J, Tao M. The effect of psychological intervention on childhood asthma. Chinese Mental Health Journal 2002;16:555‐7. [Google Scholar]
van Gaalen 2013 {published data only}
- Gaalen JL, Beerthuizen T, Meer V, Reisen P, Redelijkheid GW, Snoeck‐Stroband JB, et al. Long‐term outcomes of internet‐based self‐management support in adults with asthma: randomized controlled trial. Journal of Medical Internet Research 2013;15:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vazquez 1993 {published data only}
- Vazquez I, Buceta J. Relaxation therapy in the treatment of bronchial asthma: effects on basal spirometric values. Psychotherapy and Psychosomatics 1993;60:106‐12. [DOI] [PubMed] [Google Scholar]
Vazquez 1993a {published data only}
- Vazquez MI, Buceta JM. Psychological treatment of asthma: effectiveness of a self‐management program with and without relaxation training. Journal of Asthma 1993;30:171‐83. [DOI] [PubMed] [Google Scholar]
Wilkening 1999 {published data only}
- Wilkening R, Hartung K, Winter T. Behavioural therapeutical group program for asthmatics requiring psychological treatment. Verhaltenstherapie 1999;9:80‐1. [Google Scholar]
References to ongoing studies
ACTRN12614000915651 {unpublished data only}
- ACTRN12614000915651. Randomised controlled trial of telephone based cognitive behavioural therapy (CBT) for patients with chronic lung disease and anxiety and/or depression undergoing pulmonary rehabilitation to evaluate the effect on symptoms of anxiety and depression, quality of life, and exacerbations. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12614000915651 (registered 27 August 2014).
IRCT2015061622770N1 {unpublished data only}
- IRCT2015061622770N1. The effectiveness of mindfulness‐based cognitive therapy (MBCT) on depression, anxiety and somatic symptoms in asthma patients. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015061622770N1 (registered 6 December 2015).
NCT01583296 {published data only}
- NCT01583296. Adaptation of a behavioral treatment for Latinos with panic disorder and asthma. clinicaltrials.gov/ct2/show/NCT01583296 (first received 19 April 2012).
Additional references
Antoni 2003
- Antoni MH. Stress Management Intervention for Women with Breast Cancer. Washington, DC: American Psychological Association Press, 2003. [Google Scholar]
Asthma UK 2015
- Asthma UK. Stress and anxiety. www.asthma.org.uk/knowledge‐bank‐living‐with‐asthma‐emotional‐health‐stress‐and‐anxiety (accessed 25 February 2015).
ATS 2000
- American Thoracic Society. Proceedings of the ATS Workshop on Refractory Asthma Current Understanding, Recommendations, and Unanswered Questions. American Journal of Respiratory and Critical Care Medicine 2000;162(6):2341‐51. [DOI] [PubMed] [Google Scholar]
Avner 1988
- Avner SE, Kinsman RA. Psychologic factors and allergic diseases. In: Dierman CW, Pearlman DS editor(s). Allergic Diseases from Infancy to Adulthood. Philadelphia: WB Saunders, 1988:300‐17. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Bernardy 2013
- Bernardy K, Klose P, Busch AJ, Choy EHS, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD009796.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 1989
- Brooks CM, Richards JM, Bailey WC, Martin B, Windsor RA, Seng‐jaw S. Subjective symptomatology of asthma in an outpatient population. Psychosomatic Medicine 1989;51:102‐8. [DOI] [PubMed] [Google Scholar]
BTS/SIGN 2014
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. SIGN 141. October 2014. www.brit‐thoracic.org.uk/guidelines‐and‐quality‐standards/asthma‐guideline/ (accessed 23 February 2015).
Carr 1998
- Carr RE. Panic disorder and asthma: causes, effects and research implications. Journal of Psychosomatic Research 1998;44(1):43‐52. [DOI] [PubMed] [Google Scholar]
Cohen 1983
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior 1983;24(4):385‐96. [PubMed] [Google Scholar]
DiMatteo 2000
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine 2000;160(14):2101‐7. [DOI] [PubMed] [Google Scholar]
Ettinger 2004
- Ettinger A, Reed M, Cramer J, Epilepsy Impact Project Group. Depression and comorbidity in community‐based patients with epilepsy or asthma. Neurology 2004;63(6):1008‐14. [DOI] [PubMed] [Google Scholar]
FDA 2010
- US Food, Drug Administration. FDA Drug Safety Communication: New safety requirements for long‐acting inhaled asthma medications called Long‐Acting Beta‐Agonists (LABAs). Page Last Updated: 22 February 2010. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm (accessed 25 February 2015).
GINA 2016
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2016. http://www.ginasthma.org/ (accessed 19 May 2016).
Global Asthma Network 2014
- Global Asthma Network. The Global Asthma Report 2014. Auckland, New Zealand: Global Asthma Network, 2014. www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf (accessed 29 April 2015).
GRADEpro GDT 2016 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT 2016. Version (accessed 25 April 2016). Hamilton (ON): GRADE Working Group, McMaster University, 2016.
Heaney 2005
- Heaney LG, Robinson DS. Severe asthma treatment: need for characterising patients. Lancet 2005;365(9463):974‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huntley 2002
- Huntley A, White AR, Ernst E. Relaxation therapies for asthma: a systematic review. Thorax 2002;57:127‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyland 1995
- Hyland ME, Ley A, Fisher DW, Woodward V. Measurement of psychological distress in asthma and asthma management programmes. British Journal of Clinical Psychology 1995;34:601‐11. [DOI] [PubMed] [Google Scholar]
Juniper 1999
- Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the asthma quality of life questionnaire. Chest 1999;115(5):1265‐70. [DOI] [PubMed] [Google Scholar]
Juniper 1999a
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. European Respiratory Journal 1999;14:902‐7. [DOI] [PubMed] [Google Scholar]
Katon 2004
- Katon WJ, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosomatic Medicine 2004;66(3):349‐55. [DOI] [PubMed] [Google Scholar]
Kotses 1995
- Kotses H, Bernstein L, Bernstein DI, Reynolds RV, Korbee L, Wigal JK, et al. A self‐management program for adult asthma. Part 1: Development and evaluation. Journal of Allergy and Clinical Immunology 1995;95(2):529‐40. [DOI] [PubMed] [Google Scholar]
Lavoie 2010
- Lavoie KL, Bouthillier D, Bacon SL. Psychologic distress and maladaptive coping styles in patients with severe versus moderate asthma. Chest 137;6:1324‐31. [DOI] [PubMed] [Google Scholar]
Mancuso 2000
- Mancuso CA, Peterson MG, Charlson ME. Effects of depressive symptoms on health related quality of life in asthma patients. Journal of General Internal Medicine 2000;15:301‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Devesa 2010
- Martinez‐Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD005233.pub3] [DOI] [PubMed] [Google Scholar]
Mayo‐Wilson 2013
- Mayo‐Wilson E, Montgomery P. Media‐delivered cognitive behavioural therapy and behavioural therapy (self‐help) for anxiety disorders in adults. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD005330.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monticone 2015
- Monticone M, Cedraschi C, Ambrosini E, Rocca B, Fiorentini R, Restelli M, et al. Cognitive‐behavioural treatment for subacute and chronic neck pain. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD010664.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults: the treatment and management of depression in adults. NICE guidelines [CG90]. Published date: October 2009. www.nice.org.uk/guidance/cg90 (accessed 25 February 2015).
NICE 2011
- National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults: management in primary, secondary and community care. NICE guidelines [CG113]. Published date: January 2011. www.nice.org.uk/guidance/cg113 (accessed 25 February 2015).
NICE 2013
- National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment and treatment. NICE guidelines [CG159]. Published date: May 2013. www.nice.org.uk/guidance/cg159 (accessed 25 February 2015). [PubMed]
NIH/NHLBI 2007
- National Heart, Lung, and Blood Institute (NHLBI). Guidelines for the diagnosis and management of asthma (EPR‐3). www.nhlbi.nih.gov/health‐pro/guidelines/current/asthma‐guidelines/full‐report (accessed 15 August 2016).
Peterson 1992
- Peterson R, Reiss S. 1992. 2nd Edition. Worthington, OH: International Diagnostic Systems, Anxiety Sensitivity Index Manual. [Google Scholar]
Price 2008
- Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD001027.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2006
- Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: relationship to asthma severity and depression symptoms. Pediatrics 2006;118(3):1042‐51. [DOI] [PubMed] [Google Scholar]
Royal College of Physicians 2014
- Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry report. London: RCP, 2014. www.rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐report.pdf (accessed 23 February 2015).
Royal College of Psychiatrists 2015
- Royal College of Psychiatrists. Cognitive behavioural therapy. www.rcpsych.ac.uk/mentalhealthinfoforall/treatments/cbt.aspx (accessed 25 Februrary 2015).
Sanavio 1986
- Sanavio E, Bertolotti G, Michielin P, Vidotto G, Zotti AM. Batteria CBT 2.0 Scale Primarie: Manuale. Firenze: Organizzazione Speciali, 1986. [Google Scholar]
Shavitt 1992
- Shavitt RG, Gentil V, Mandetta R. The association of panic/agoraphobia and asthma: contributing factors and clinical implications. General Hospital Psychiatry 1992;14(6):420‐3. [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
Tellegen 1988
- Tellegen A, Lykken DT, Bouchard TJ, Wilcox KJ, Segal NL, Rich S. Personality similarity in twins reared apart and together. Journal of Personality and Social Psychology 1988;54:1031‐9. [DOI] [PubMed] [Google Scholar]
Thoren 2000
- Thoren CT, Petermann F. Reviewing asthma and anxiety. Respiratory Medicine 2000;94(5):409‐15. [DOI] [PubMed] [Google Scholar]
White 2001
- White CA. Cognitive behavioural principles in managing chronic disease. Western Journal of Medicine 2001;175(5):338‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 1993
- Wigal JK, Stout C, Brandon M, Winder JA, McConnaughy K, Creer TL, et al. The Knowledge, Attitude, and Self‐Efficacy Asthma Questionnaire. Chest 1993;104(4):1144‐8. [DOI] [PubMed] [Google Scholar]
Yorke 2006
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD002982.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yorke 2011
- Yorke J, Russell A, Swigris J, Shuldham C, Haigh C, Jones PW. Assessment of dyspnoea in asthma: validation of the Dyspnoea‐12. Journal of Asthma 2011;48(6):602‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zielinski 2000
- Zielinski TA, Brown ES, Nejtek VA, Khan DA, Moore JJ, Rush AJ. Depression in asthma: prevalence and clinical implications. Primary Care Companion to The Journal of Clinical Psychiatry 2000;2(5):153‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kew 2015
- Kew KM, Yorke J, Nashed M. Cognitive behavioural therapy (CBT) versus usual care for adults and adolescents with asthma. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011818] [DOI] [PMC free article] [PubMed] [Google Scholar]


