Abstract
Background
Inflammation and oedema of the facial nerve are implicated in causing Bell's palsy. Corticosteroids have a potent anti‐inflammatory action that should minimise nerve damage. This is an update of a review first published in 2002 and last updated in 2010.
Objectives
To determine the effectiveness and safety of corticosteroid therapy in people with Bell's palsy.
Search methods
On 4 March 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and LILACS. We reviewed the bibliographies of the randomised trials and contacted known experts in the field to identify additional published or unpublished trials. We also searched clinical trials registries for ongoing trials.
Selection criteria
Randomised trials and quasi‐randomised trials comparing different routes of administration and dosage schemes of corticosteroid or adrenocorticotrophic hormone therapy versus a control group receiving no therapy considered effective for this condition, unless the same therapy was given in a similar way to the experimental group.
Data collection and analysis
We used standard Cochrane methodology. The main outcome of interest was incomplete recovery of facial motor function (i.e. residual facial weakness). Secondary outcomes were cosmetically disabling persistent sequelae, development of motor synkinesis or autonomic dysfunction (i.e. hemifacial spasm, crocodile tears) and adverse effects of corticosteroid therapy manifested during follow‐up.
Main results
We identified seven trials, with 895 evaluable participants for this review. All provided data suitable for the primary outcome meta‐analysis. One of the trials was new since the last version of this Cochrane systematic review. Risk of bias in the older, smaller studies included some unclear‐ or high‐risk assessments, whereas we deemed the larger studies at low risk of bias. Overall, 79/452 (17%) participants allocated to corticosteroids had incomplete recovery of facial motor function six months or more after randomisation; significantly fewer than the 125/447 (28%) in the control group (risk ratio (RR) 0.63, 95% confidence interval (CI) 0.50 to 0.80, seven trials, n = 895). The number of people who need to be treated with corticosteroids to avoid one incomplete recovery was 10 (95% CI 6 to 20). The reduction in the proportion of participants with cosmetically disabling sequelae six months after randomisation was very similar in the corticosteroid and placebo groups (RR 0.96, 95% CI 0.40 to 2.29, two trials, n = 75, low‐quality evidence). However, there was a significant reduction in motor synkinesis during follow‐up in participants receiving corticosteroids (RR 0.64, 95% CI 0.45 to 0.91, three trials, n = 485, moderate‐quality evidence). Three studies explicitly recorded the absence of adverse effects attributable to corticosteroids. One trial reported that three participants receiving prednisolone had temporary sleep disturbances and two trials gave a detailed account of adverse effects occurring in 93 participants, all non‐serious; the combined analysis of data from these three trials found no significant difference in adverse effect rates between people receiving corticosteroids and people receiving placebo (RR 1.04, 95% CI 0.71 to 1.51, n = 715).
Authors' conclusions
The available moderate‐ to high‐quality evidence from randomised controlled trials showed significant benefit from treating Bell's palsy with corticosteroids.
Plain language summary
Corticosteroids for Bell's palsy
Review question
What are the effects of corticosteroids on Bell's palsy?
Background
Bell's palsy is a paralysis or weakness of muscles in the face, usually on one side, with no certain cause. Symptoms usually recover, although not always. Reducing inflammation of the facial nerve using corticosteroid medicines (steroids) is thought to limit nerve damage. This is an update of a review first published in 2002 and last updated in 2010.
Study characteristics
We identified seven clinical trials involving 895 people with one‐sided mild, moderate or severe Bell's palsy of unknown cause. All of these trials reported rates of incomplete recovery (the proportion of people left with facial weakness) and we were able to combine the results. People in the studies were aged from 2 to 84 years. They were treated with corticosteroids or placebo (inactive treatment), either alone or in combination with other therapies. One trial only involved children, from 24 months to 74 months old. The duration of the included studies for adults and children ranged from 157 days to 12 months.
Key results and quality of the evidence
Incomplete recovery
According to moderate quality to high quality evidence, corticosteroids reduced the number of people left with facial weakness after Bell's palsy compared to placebo (a pretend medicine). This finding was based on data from seven studies involving 895 participants with Bell's palsy of varying degrees of severity. We calculated that to stop one person from being left with facial weakness, 10 people need to be treated.
Five studies provided data on long‐term after‐effects of Bell's palsy following treatment. Two of the studies (75 participants) looked at persistent effects on facial appearance after six months or more. The effect was nearly the same for corticosteroids and placebo, showing that participants who had corticosteroids benefited slightly, although this evidence was low quality. Data from three studies (485 participants) showed clearly that people who received corticosteroids developed less motor synkinesis (unwanted facial movements) and crocodile tears (watery eyes when eating or chewing), compared with people who received placebo alone. This finding was based on moderate‐quality evidence.
Side effects
Three studies reported that no side effects could be attributed to corticosteroid treatment. Based on moderate‐quality evidence from three studies (715 participants), numbers experiencing side effects were similar with corticosteroids and placebo.
The evidence is current to March 2016.
Summary of findings
Summary of findings for the main comparison. Corticosteroids compared to placebo or no treatment for Bell's palsy.
| Corticosteroids compared to placebo or no treatment for Bell's palsy | ||||||
| Patient or population: people with Bell's palsy Settings: primary and secondary care Intervention: corticosteroids Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Corticosteroids | |||||
| Incomplete recovery ≥ 6 months after randomisation House‐Brackmann grading system and Sunnybrook scale Follow‐up: 157 days to 12 months | 281 per 1000 | 177 per 1000 (140 to 225) | RR 0.63 (0.50 to 0.80) | 895 (7 studies) | ⊕⊕⊕⊕ high1 | NNTB 10, 95% CI 6 to 20 |
| Cosmetically disabling persistent sequelae ≥ 6 months after randomisation Clinical assessment Follow‐up: mean 6 months | 216 per 1000 | 208 per 1000 (86 to 495) | RR 0.96 (0.4 to 2.29) | 75 (2 studies) | ⊕⊕⊝⊝ low2,3 | ‐ |
| Motor synkinesis and crocodile tears Clinical assessment Follow‐up: 9 to 12 months | 260 per 1000 | 167 per 1000 (117 to 237) | RR 0.64 (0.45 to 0.91) | 485 (3 studies) | ⊕⊕⊕⊝ moderate4 | ‐ |
| Adverse effects Follow‐up: 9 to 12 months | 127 per 1000 |
133 per 1000 (91 to 192) |
RR 1.04 (0.71 to 1.51) | 715 (3 studies) | ⊕⊕⊕⊝ moderate5 | 3 other studies recorded that no adverse effects occurred with corticosteroid treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Two trials excluded participants who were found to have clinical evidence of herpes zoster infection (Lagalla 2002; Taverner 1954). In addition, two studies did not use a scoring system such as the House‐Brackmann or Sunnybrook scale to assess facial motor function, relying upon clinical examination, electromyographic tests or photographs (May 1976; Taverner 1954). Taverner 1954 reported outcomes at five months rather than at six months or more. However, we felt that these limitations did not compromise the generalisability of the findings.
2 We downgraded twice: first for imprecision ‐ there was a low number of events and pooled RR allowed the possibility of both no effect and the chance of harm; second for publication bias ‐ of the seven included studies, five did not provide data on the presence of cosmetically disabling sequelae six months or more after randomisation.
3 We downgraded once for imprecision. There was a low number of events.
4 We downgraded the quality of the evidence to moderate because of publication bias.
5 We downgraded for publication bias ‐ only three of seven studies provided data on adverse effects.
Background
Description of the condition
Bell's palsy is an acute, generally unilateral, paralysis or weakness of facial musculature consistent with peripheral facial nerve dysfunction, of no detectable cause (Niparko 1993). Additional symptoms frequently include pain around or behind the ear sometimes extending into the occipital or cervical region. Impaired tolerance to ordinary levels of noise and disturbed sense of taste on the same side may also be present (Burgess 1984).
Epidemiological studies have reported an annual incidence of 23 to 25 per 100,000 per year (Martyn 1997; Victor 1994), but one study using a general practitioner (GP) database has suggested it may be even higher at 37 per 100,000 per year (Morales 2013). Bell's palsy affects men and women more or less equally. Until recently was thought to be most common in the 30‐ to 45‐year age group (Bateman 1992; Brandenberg 1993; Katusic 1986; Peitersen 1982; Peitersen 2002; Yanagihara 1988), although the one more recent primary care database study suggested a peak in people over 70 years of age (Morales 2013). The condition presents disproportionately among pregnant women and people who have diabetes, influenza, a cold, or some other upper respiratory ailment. On average, every year a British GP will see one or two people who have developed the condition. Both sides of the face are affected equally often (Prescott 1988).
The aetiology of Bell's palsy is still debated. A viral infection, vascular ischaemia, autoimmune inflammatory disorders and heredity have been proposed as underlying causes (Adour 1982; Burgess 1984; Lackner 2010). A viral aetiology has gained popularity since the isolation of herpes simplex virus‐1 (HSV‐1) genome from the facial nerve endoneurial fluid of people with Bell's palsy (Lackner 2010; Murakami 1996). The prognosis is on the whole favourable. One of the largest series of people with Bell's palsy including those who were not receiving specific therapy, showed that 85% of people with Bell's palsy began to recover within three weeks of onset (Peitersen 1982). For the remaining 15%, partial recovery occurred three to six months later. The same series showed that normal facial expression reappeared in 71% of cases, 13% had insignificant sequelae, and the remaining 16% had permanently diminished function with aberrant innervation (expressed as motor synkinesis or autonomic dysfunction) and post‐paralytic spasms.
Description of the intervention
The treatment of Bell's palsy used to be highly controversial. Corticosteroids were the treatment of choice, based mainly on non‐randomised comparisons (Adour 1972). The authors of numerous clinical series both espoused and condemned corticosteroid therapy with, what appeared to them, equally convincing arguments (Burgess 1984). Four systematic reviews found a significant trend favouring corticosteroid treatment in improving the recovery of facial motor function (de Almeida 2009; Grogan 2001; Ramsey 2000; Williamson 1996). However, their conclusions were affected by including trials of poor quality in the pooled estimate. de Almeida 2009, a state‐of‐the‐art, high‐quality systematic review, differed from our methodology in the way they collected data, in the inclusion of a non‐randomised trial that we excluded (Martinez 1990), and in the exclusion of a trial that we included in our analysis (Taverner 1954).
How the intervention might work
Corticosteroids are known to have an anti‐inflammatory mode of action, which reduces oedema and inflammation of the facial nerve in the acute presentation of Bell's palsy.
Why it is important to do this review
The purpose of this systematic review was to collect and analyse the available evidence from randomised controlled trials concerning the use of corticosteroids for improving the outcome of people with Bell's palsy. This is an update of a review first published in 2002 and last updated in 2010. We updated the review to assess the quality of the evidence regarding the benefit of corticosteroids in Bell's palsy using current methodology, integrate any new evidence and to determine whether further research would be likely to change the estimate of effect or its certainty.
Objectives
To determine the effectiveness and safety of corticosteroid therapy in people with Bell's palsy.
Methods
Criteria for considering studies for this review
Types of studies
Trials of the use of corticosteroid therapy for the treatment of recent‐onset Bell's palsy in which an attempt had apparently been made to conduct a randomised or quasi‐randomised comparison between corticosteroid therapy and a placebo, or open control group. As in the previous version of the review, we used study quality as an exclusion criteria excluding trials with a high risk of bias in several domains.
Types of participants
Participants of any age with clinically diagnosed Bell's palsy, irrespective of the time of evolution of symptoms. We included all participants considered to have Bell's palsy or acute idiopathic facial nerve paralysis by the study authors, irrespective of the ancillary studies they performed to rule out other causes of facial paralysis. We included in the analysis all participants considered eligible by the authors of the studies, irrespective of associated conditions.
Types of interventions
We included trials using any corticosteroid or adrenocorticotrophic hormone therapy, irrespective of the route of administration (oral or parenteral) and length of therapy. We excluded trials in which a drug considered 'effective' for this condition was given to the control group, unless it was given in a similar way to the experimental group. The exception was studies in which control and treatment groups received concomitant antiviral therapy; these are not included in this review, but in the updated Cochrane review 'Antiviral treatment for Bell's palsy (idiopathic facial paralysis)' (Gagyor 2015).
We excluded trials comparing different types of corticosteroids or different dosage schemes, unless the trial included a placebo group.
Types of outcome measures
Primary outcomes
Incomplete recovery of facial motor function six months or more after randomisation.
Secondary outcomes
Cosmetically disabling persistent sequelae of facial paralysis (poor recovery of facial motor function as defined by the authors of the studies) six months or more after randomisation.
Motor synkinesis or autonomic dysfunction during follow‐up (including facial spasm, motor synkinesis and crocodile tears).
Adverse effects attributable to the use of corticosteroids.
Search methods for identification of studies
Electronic searches
On 4 March 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL in the Cochrane Register of Studies Online), MEDLINE (January 1966 to February 2016), EMBASE (January 1980 to February 2016) and LILACS (January 1982 to February 2016). The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register (Appendix 1), CENTRAL (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4) and LILACS (Appendix 5).
On 21 June 2016, we searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (who.int/trialsearch/) for ongoing studies. We searched both using the term "Bell's Palsy".
Searching other resources
We reviewed the bibliographies of the randomised trials and contacted the study authors and known experts in the field to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
Six review authors scrutinised published and unpublished papers retrieved by the literature searches to select papers for inclusion. At least two review authors independently assessed each paper for relevance, eligibility and quality. There were no disagreements about inclusion. We applied no language limitations.
Data extraction and management
Review authors collected and analysed data in pairs (FG and VM, DS and MS, IG and FS). We collected data on the following.
Methods: study design, study duration, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number (n), mean age, age range, gender, severity of condition, diagnostic criteria, baseline characteristics, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported; definition of recovery status.
Notes: funding for trial and notable conflicts of interest of trial authors.
All six review authors had a selection of papers to read, review for quality and extract data from. At least two review authors assessed each trial. All review authors agreed data extraction.
Two review authors (IG and VM) inputted data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
IG completed the 'Risk of bias' table. FS and FD individually reviewed the 'Risk of bias' assessments. We assessed bias by scoring studies as at high, low or unclear risk of bias using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
When comparing studies using different symptom scores to assess outcomes, we used the House‐Brackmann scale when available as this was the most widely used or had comparisons to other scales available. When assessing adverse effects, we reported the number of participants affected, as opposed to the number of events, to facilitate data comparison.
We used dichotomous outcomes and we analysed data as risk ratios (RR) with a corresponding 95% confidence interval (CI). We calculated a treatment effect using the Mantel‐Haenszel method (Egger 2007).
Unit of analysis issues
Where a single trial included multiple trial arms, we included only arms relevant to this review. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) had been combined in the same meta‐analysis, our preference would be to combine groups to create a single comparison if appropriate, or otherwise use one of the other approaches described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
One of the trial authors, who is also a review author (FS), provided unpublished trial data. We also contacted a previous study team member (P Lockhart) for additional information on other studies and received a response. We either extracted the number of participants who completed a treatment originally allocated and the number with the outcome, and considered the potential effect of missing data when presenting results, or reported intention‐to‐treat analyses as presented in trial reports. Where data were unavailable we contacted the original authors, when there was no response, we calculated the numbers from the rates and percentages presented in the papers. We undertook no sensitivity analyses for missing values. For some trials, we calculated either number in the analysis or the number experiencing an event using the percentage experiencing an event.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis, using the thresholds in the Cochrane Handbook for Systematic Reviews of Interventions as guidance (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We interpreted the I2 statistic in the context of its CI, the Chi2 test, P value and the size of the effect.
Assessment of reporting biases
Since we were unable to pool more than 10 trials, we could not create a funnel plot to explore possible small‐study biases.
Data synthesis
We used a fixed‐effect model for our analysis and performed a sensitivity analysis with a random‐effects model.
If the review had included more than one comparison that could not be included in the same analysis, we would have reported the results for each comparison separately.
Throughout we have utilised the following notations:
OS: corticosteroid treatment alone
OO: placebo or no treatment only
Using these notations, we conducted the following comparison: OS versus OO.
'Summary of findings' table
At this update, we added a 'Summary of findings' table. We used the following outcomes: incomplete recovery, cosmetically disabling persistent sequelae six months or more after randomisation, motor synkinesis and crocodile tears, and adverse effects.
Under the GRADE approach, the levels of quality of evidence are high, moderate, low or very low. Assessors start at high quality and may downgrade the evidence for the pre‐specified outcomes depending on five GRADE considerations, which are: study limitations, consistency of effect, imprecision, indirectness and publication bias. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADEpro software (GRADEpro 2008). We have justified all decisions to downgrade or upgrade the quality of studies using footnotes and have made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses for this review. The protocol detailed possible subgroup analyses, although none were appropriate for the included studies.
Sensitivity analysis
We carried out the following sensitivity analyses.
Repeated the analyses excluding smaller and underpowered studies.
Repeated the analyses excluding studies with short‐term follow‐up (less than nine months).
Results
Description of studies
Results of the search
In the updated searches for this review, we found 59 references in the Cochrane Neuromuscular Specialised Register, 81 in CENTRAL, 818 in MEDLINE, 117 in EMBASE and 15 in LILACS.
There were seven completed randomised controlled trials comparing corticosteroids with no active control when this review was first written. In subsequent updates, review authors identified five other potentially relevant trials (Austin 1993; Bento 1991; Engström 2008; Lagalla 2002; Sullivan 2007).
In all, seven trials including 895 evaluable participants met our inclusion criteria (Austin 1993; Engström 2008; Lagalla 2002; May 1976; Sullivan 2007; Taverner 1954; Unuvar 1999).
See Figure 1 for a flow chart illustrating the study selection process for this update.
1.
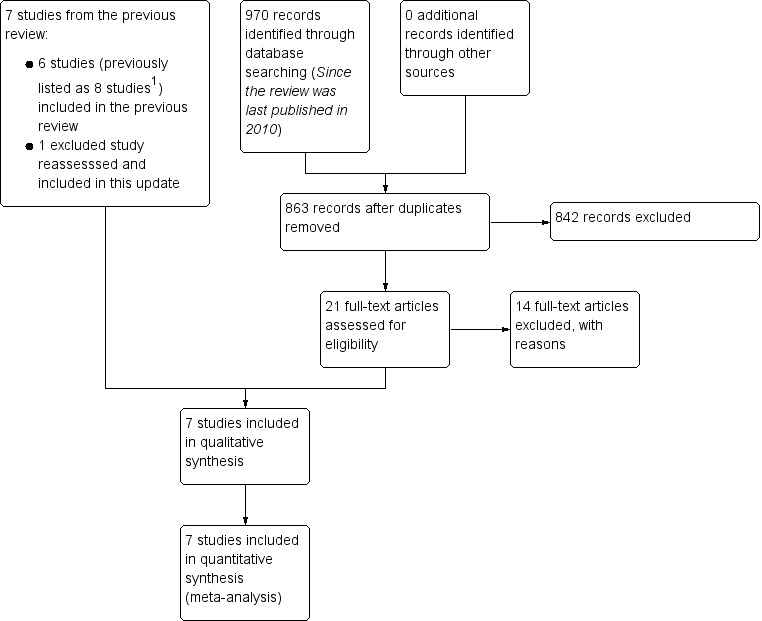
Study flow diagram illustrating the study selection process for this update.
1. The previous version of this review listed comparisons involving participants treated with antiviral therapy from Sullivan 2007 and Engström 2008 as two additional separate studies. These two comparisons are not included in this update.
The previous update of this review, Salinas 2010, listed seven excluded studies (Akpinar 1979; Austin 1993; Bento 1991; Bento 1994; Brown 1982; Martinez 1990; Wolf 1978). We have linked two references previously listed as separate trials (Bento 1991 and Bento 1994) under one trial identifier in this update (Bento 1991). We included Austin 1993, previously excluded because of potential attrition bias, as current practice is to include all trials eligible according to the selection criteria.
Authors of the last version of this review included groups receiving antiviral therapy and considered the different comparisons from Engström 2008 and Sullivan 2007 as separate studies (Engström 2008b and Sullivan 2007b). We did not consider the two antiviral‐treated participant groups from those trials in this updated review. These comparisons are included in the companion Cochrane review, 'Antiviral treatment for Bell's palsy (idiopathic facial paralysis)' (Gagyor 2015). In this update, we included only the comparisons of corticosteroid versus placebo or corticosteroids versus no treatment.
Included studies
Summary details of the included trials are given in Characteristics of included studies.
Austin 1993 recruited 107 participants whose symptoms had occurred within five days of presentation to the study centre. Of these, 31 participants did not return for initial follow‐up. The 76 remaining participants subsequently entered a double‐blinded randomised controlled study allocating them to a corticosteroid treatment group (prednisone) or a placebo group. The trial authors gave no details about binding or randomisation. Assessment of participants occurred within five days of onset and at "regular intervals until they experienced recovery from their acute paralysis". Final follow‐up was at six months after recovery. The trial authors stated, "to be included in the analysis, patients must have completed follow up until their acute recovery occurred." Investigators measured disease status using the House‐Brackmann grading system. At first evaluation, participants underwent various tests including blood tests, an audiogram, a nerve excitability test using the maximal stimulation technique and, if indicated, electroneurography. Treatment with prednisone was started at 30 mg twice daily for the first five days followed by a reducing dose stated by the trial authors.
The study reported final outcomes on 53 participants at six months after recovery. The primary outcome was time to recovery, for which trial authors found no statistically significant difference between the two groups. Secondary outcomes included facial paralysis grade at onset versus grade at recovery. There was a statistically significant difference in incomplete recovery rates favouring the treatment group: 5/23 participants receiving prednisone had incomplete recovery at six months versus 10/30 participants receiving placebo (RR 0.65, 95% CI 0.26 to 1.65). Investigators found no significant difference in persistence of pain during recovery, with 3/23 participants in the prednisone group still experiencing pain at six months, compared with 4/30 participants in the placebo group (RR 0.98, 95% CI 0.24 to 3.95).
Engström 2008 had a factorial design that randomised 829 participants into four treatment groups using a two‐stage computerised process. The four treatment groups were prednisolone with placebo, prednisolone with valaciclovir, valaciclovir with placebo, and double placebo. Treatment started within 72 hours of symptom onset. The trial was double‐blind (administrator and participant) for assessment of recovery status until the end of follow‐up. Participants were assessed at onset, after two weeks (11 to 17 days) and after 1, 2, 3, 6 and 12 months. Trialists measured disease status using the House‐Brackmann grading system and the Sunnybrook scale, defining complete recovery status as a Sunnybrook score of 100 and a House‐Brackmann grade of I. Data analysis included an assessment of treatment interaction.
The study reported a positive effect on recovery time due to prednisolone (comparing recovery rates at 12 months in the prednisolone group with the placebo group). For this review, we analysed the corticosteroid‐placebo combination (OS) versus the double placebo combination (OO) 12 months after the onset of facial palsy. Using the Sunnybrook definition, 50/210 participants had an incomplete recovery with prednisolone compared with a greater proportion (73/206) in the placebo group (RR 0.67, 95% CI 0.50 to 0.91).
Lagalla 2002 randomised 62 participants within three days of onset of Bell's palsy to high‐dose prednisone, 1 g daily for three days, then 0.5 g daily for three days, administered intravenously, using saline solution as a placebo. The age range was 15 to 84 years. Exclusion criteria included peptic ulcer disease, pregnancy, severe hypertension, other neurological conditions, diseases of the ear and previous treatment. As in Taverner 1954, investigators excluded participants with herpes zoster oticus from the analyses (four of the participants initially randomised in Lagalla 2002). The investigators monitored participants for 12 months. They assessed recovery of facial motor function using the House‐Brackmann scale. The trial authors reported the development of disabling synkinesis at 12‐month follow‐up.
Lagalla 2002 reported final outcomes on 58 participants at 12 months. In the prednisolone group, 5/30 participants had incomplete recovery, compared with 8/28 participants in the placebo group (RR 0.58, 95% CI 0.22 to 1.57).
May 1976 included 51 participants within two days of Bell's palsy onset and reported outcomes using clinical assessment and photographic documentation without a validated scoring system. The trial report did not specify the age range of participants in this double‐blinded study. In the intervention group, participants received prednisone 410 mg in descending doses over 10 days. The placebo intervention was vitamins. Trialists excluded people with chronic otitis media, trauma, loss of lacrimation, or recurrent or bilateral palsy.
May 1976 was the only study that stated that there was no benefit from corticosteroids for the recovery of Bell's palsy (RR 1.16, 95% CI 0.57 to 2.36).
Sullivan 2007 recruited 551 participants to be treated within 72 hours of onset. Participants were randomised by a dedicated remote telephone‐computerised mechanism in a two‐stage process into four treatment groups in a factorial design: prednisolone with placebo, prednisolone with aciclovir, aciclovir with placebo or double placebo. Participants received prednisolone 25 mg twice daily for 10 days. The trial was blinded for administrator, participant and assessment of recovery status until the end of follow‐up. Assessments took place at onset, after three months and, if participants were still unwell, again after nine months. The investigators measured recovery status on the House‐Brackmann scale, with complete recovery defined as House‐Brackmann grade I. Data analysis included an assessment of treatment interaction.
Sullivan 2007 reported final outcomes on 496 completed participants at three and nine months. In the corticosteroid (OS) group, 5/127 participants had incomplete recovery, compared with 18/122 participants in the placebo (OO) group (RR 0.27, 95% CI 0.10 to 0.70).
Taverner 1954 was a double‐blind trial that included 26 participants with a range of ages from 12 to 76 years within 10 days of onset of Bell's palsy. Participants received either oral cortisone acetate 1 g in descending doses over eight days or placebo tablets. Participants were monitored for up to 157 days. The trial excluded participants with other neurological conditions or diseases of the ear. Taverner 1954 included participants with herpes zoster oticus. These participants were allocated in equal numbers to each treatment arm, and trial authors excluded them from the analyses. The trialists did not used a validated scoring system to measure outcomes.
Taverner 1954 used the recovery of facial motor function as the main outcome measure and reported a small, non‐significant benefit of corticosteroids compared with placebo; CIs allowed for effects in either direction (RR 0.86, 95% CI 0.27 to 2.71).
Unuvar 1999 conducted a non‐blinded, randomised controlled trial on children with severe to complete presentation of Bell's palsy (House‐Brackmann grades IV to V). They randomised 42 children with an age range of 24 to 74 months using a computer‐generated random sequence into two groups. All participants entered the trial within three days of onset of Bell's palsy and received either methylprednisolone 1 mg/kg daily for 10 days then gradually withdrawn over three to five days or no specific treatment. They excluded participants with other neurological conditions, diseases of the ear or systemic diseases. The primary outcome was the recovery of facial motor function measured on the House‐Brackmann facial grading system.
Unuvar 1999 reported a benefit from the treatment with corticosteroids although wide CIs allowed for the possibility of both no effect and a large effect (RR 0.14, 95% CI 0.01 to 2.61).
Ongoing studies
We identified one trial report on the World Health Organization International Clinical Trials Registry Platform search portal (Babl 2015). From the website, it appeared that the study has now started recruitment; we will assess this trial fully in future review updates. See Characteristics of ongoing studies.
Excluded studies
Akpinar 1979 compared three groups of 10 participants each; two groups received different dosage regimens of corticosteroids, and the other received placebo. It was unclear who diagnosed the participants with Bell's palsy and there were varying dosage regimens of corticosteroids used. Two studies were not randomised or quasi‐randomised (Brown 1982; Martinez 1990). It was not possible to extract complete information on the specified outcomes for one study (Wolf 1978). Finally, we excluded Bento 1991 as number of participants and outcome events by treatment groups was not provided; in addition, 50% of the participants were lost to follow‐up.
Risk of bias in included studies
Figure 2 summarises the 'Risk of bias' assessment for each included study.
2.
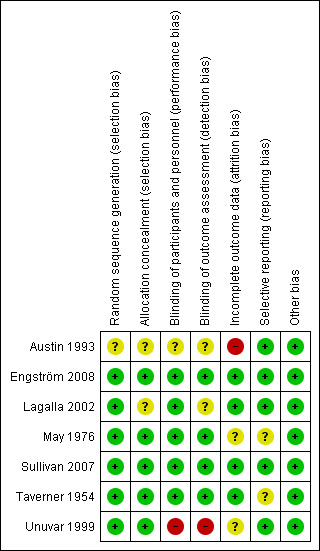
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.
Allocation
All included trials reported the method of randomisation. Four used randomisation centralised at a pharmacy, in accordance with a master sheet of random numbers (May 1976; Taverner 1954), or an automated permuted block technique (Engström 2008; Sullivan 2007). One study used a random number list (Lagalla 2002), and another used a computer‐based program (Unuvar 1999). It was not clear from the reports if there was adequate allocation concealment in Lagalla 2002 or Unuvar 1999. We considered none of the trials to be at high risk of bias for random sequence generation or allocation concealment.
Blinding
Five trials were double‐blind, using placebo in the control group (Engström 2008; Lagalla 2002; May 1976; Sullivan 2007; Taverner 1954). Two of these trials used lactose as the placebo (Sullivan 2007; Taverner 1954), one used vitamins (May 1976), and one used a saline solution (Lagalla 2002). Two trials did not specify what was used as placebo, but they stated that this was formulated to have the same size, smell and colour (Engström 2008; Unuvar 1999). In the trial using vitamins as placebo, the experimental group received similar looking capsules containing prednisone plus vitamins. The trial using saline solution as placebo gave intramuscular vitamins to all participants for 15 days (Lagalla 2002). In one trial, it was not clear from the report if the physicians administering the drug were blinded (Lagalla 2002). There were no serious imbalances in baseline prognostic factors between groups.
Incomplete outcome data
Engström 2008, Sullivan 2007; and Taverner 1954 had drop‐out rates of less than 10%. No participants dropped out in May 1976. Lagalla 2002 included drop‐outs in analyses on an intention‐to‐treat basis, by assuming that drop‐outs had a poor outcome. Austin 1993 had a drop‐out rate of 50% for outcomes six months after recovery. Unuvar 1999 did not report on attrition.
Selective reporting
All studies reported their intended primary outcomes. Engström 2008 reported all primary outcomes, with secondary outcomes reported in subsequent publications (Axelsson 2012; Berg 2012).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
As all trials reported different intervals and lengths of follow‐up, we performed analyses on data reported at the end of the study periods. These were 157 days (Taverner 1954), six months (May 1976; Austin 1993), nine months (Sullivan 2007), and 12 months (Engström 2008; Lagalla 2002; Unuvar 1999).
Primary outcome
Incomplete recovery of facial motor function six months or more after randomisation
All seven trials, with 895 participants, provided data for the outcome complete recovery of facial motor function at six months' follow‐up or more (we included data from Taverner 1954, which reported at five months). The number of people with incomplete recovery at six months' follow‐up was lower in the corticosteroid group compared to the control group (RR 0.63, 95% CI 0.50 to 0.80) (Analysis 1.1; Figure 3).
1.1. Analysis.
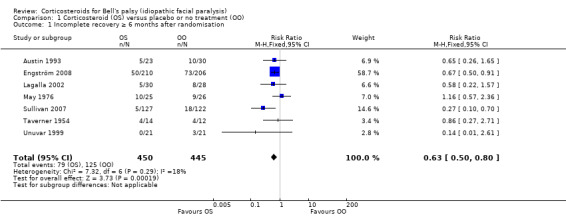
Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 1 Incomplete recovery ≥ 6 months after randomisation.
3.
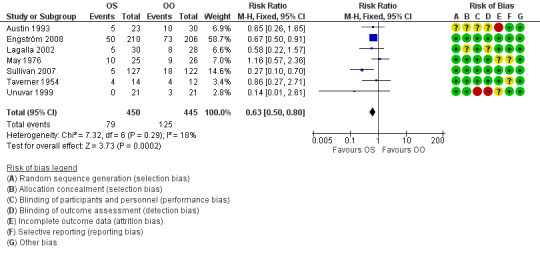
Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.1 Incomplete recovery ≥ 6 months after randomisation.
The tests for statistical heterogeneity were marginally significant (Chi2 = 7.32, P value = 0.29, I2 = 18%). This small degree of heterogeneity was due to the differences in the findings between the large studies (Engström 2008; Sullivan 2007), and small, underpowered studies (Austin 1993; Lagalla 2002; May 1976; Taverner 1954; Unuvar 1999). There were better outcomes in two of the studies (Engström 2008; Sullivan 2007).
The number of people who need to be treated with steroids to avoid one person with incomplete recovery was 10 (95% CI 6 to 20).
Sensitivity analyses
We removed smaller underpowered studies (Austin 1993; Lagalla 2002; May 1976; Taverner 1954; Unuvar 1999) from the main analysis leaving the two largest studies (Engström 2008; Sullivan 2007). This analysis confirmed the size and direction of effect, but with higher statistical heterogeneity (RR 0.59, 95% CI 0.44 to 0.79, Chi2 = 3.32, I2 = 70%).
We analysed the main outcome for the studies that had the longest follow‐up (i.e. between nine and 12 months) (Engström 2008; Lagalla 2002; Sullivan 2007). This did not significantly change the result.
Secondary outcomes
Cosmetically disabling persistent sequelae six months or more after randomisation
Two trials with 75 participants provided data on the number of participants with severe paralysis, or what may be judged as cosmetically disabling sequelae, at six months' follow‐up or more (May 1976; Taverner 1954). When we combined the data, the number of participants with cosmetically disabling sequelae was similar in the corticosteroid group and the control group (RR 0.96, 95% CI 0.40 to 2.29) (Analysis 1.2; Figure 4)
1.2. Analysis.
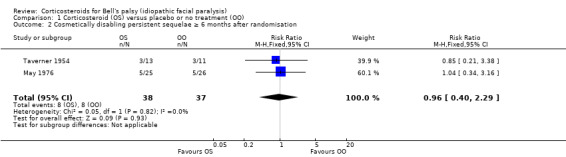
Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.
4.
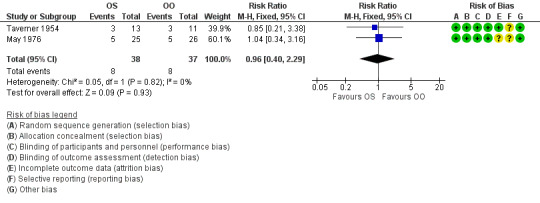
1.2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.
Motor synkinesis or crocodile tears during follow‐up
Three trials, with 485 evaluable participants, reported separate data on motor synkinesis or crocodile tears during follow‐up (Austin 1993; Engström 2008; Lagalla 2002). Two trials reported the occurrence of disabling synkinesis at 12 months' follow‐up (Engström 2008; Lagalla 2002); the other trial reported at six months' follow‐up (Austin 1993). After pooling these data, we found a significant reduction in the number of people with motor synkinesis in the corticosteroid group (40/241) compared to the placebo group (64/248) (RR 0.64, 95% CI 0.45 to 0.91) (Analysis 1.3; Figure 5). We found no statistical heterogeneity (Chi2 = 0.42, df = 2 (P = 0.81), I2 = 0%).
1.3. Analysis.
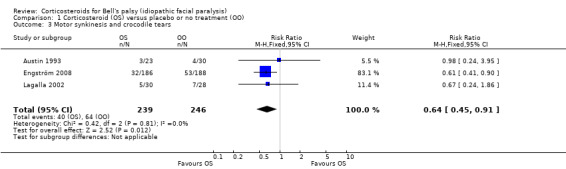
Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 3 Motor synkinesis and crocodile tears.
5.
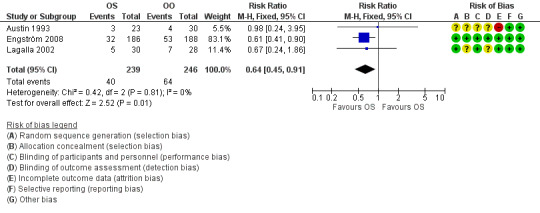
Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.3 Motor synkinesis and crocodile tears.
Adverse effects attributable to the use of corticosteroids
Three studies explicitly recorded the absence of adverse effects attributable to the experimental treatment (May 1976; Taverner 1954; Unuvar 1999). One trial reported that three participants receiving prednisone had temporary sleep disturbances (Lagalla 2002). Another reported "no severe side effects of steroid therapy", although there were single reported cases of mood swings, dyspepsia and minor conjunctivitis by individual participants (Austin 1993). Two trials gave a detailed account of 93 adverse effects (Engström 2008; Sullivan 2007), all of them non‐serious, with no significant difference between people receiving corticosteroids and people receiving placebo (RR 1.04, 95% CI 0.71 to 1.51). Three deaths occurred; all were deemed to be unrelated to treatment, all were in the groups not receiving prednisolone. One man with a history of recurrent atrial fibrillation had a transient relapse while taking prednisolone and valaciclovir (Figure 6).
6.
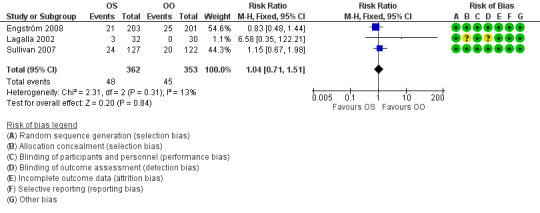
Forest plot of comparison: 1 Incomplete recovery of facial motor function, outcome: 1.4 Adverse effects.
Subgroup analyses
In this updated version of the review, we did not perform any subgroup analyses.
Discussion
Summary of main results
High‐quality evidence from randomised controlled trials indicated benefit from the use of corticosteroids in Bell's palsy.
Two trials reached a statistically significant difference favouring the use of prednisolone (Engström 2008; Sullivan 2007). Meta‐analysis of the complete high‐quality randomised controlled trial literature convincingly supported a beneficial effect of prednisolone for reducing the numbers of participants with incomplete recovery.
Moderate‐quality evidence indicated that participants who received corticosteroid treatment had less motor synkinesis and crocodile tears than the placebo group.
Based on these two studies, low‐quality evidence revealed no important differences between corticosteroids and placebo in cosmetically disabling persistent sequelae, but wide CIs allowed for the possibility of an effect in either direction.
The included studies did not report differences in the occurrence of adverse effects between corticosteroids and placebo and additionally no serious adverse effects were reported. However, corticosteroids have well‐known adverse effects, of which the incidence rises markedly with prolonged dosages above 10 mg of prednisolone or its equivalent per day (Dollery 1999). Usually corticosteroid courses in Bell's palsy are short and the dose quickly reduces, making the likelihood of adverse effects in practical use less than in longer‐term indications.
Overall completeness and applicability of evidence
No new studies have been published since the previous Cochrane review (Salinas 2010). The two most recent, well‐powered studies (Engström 2008; Sullivan 2007), with five smaller studies (Austin 1993; Lagalla 2002; May 1976; Taverner 1954; Unuvar 1999), provided a significant result based on the evidence provided. These studies sufficiently address the objectives of this review in that they investigate all relevant participants, interventions and outcomes. The findings are in accordance with suggested current clinical practice (Madhok 2009).
Quality of the evidence
For the main outcome of this review (incomplete recovery six months or more after randomisation), all seven studies combined provided high‐quality evidence. In addition, for motor synkinesis and crocodile tears, data from three studies provided moderate‐quality evidence. Cosmetically disabling persistent sequelae six months or more after randomisation was the only analysis performed where the quality of evidence was low. For adverse effects, the evidence was moderate quality. Reasons for downgrading the quality of evidence were that in one study participants and assessors were not blinded to the treatment that they received (Unuvar 1999), and in another study, loss to follow‐up was 50%, representing incomplete outcome data (Austin 1993). Two studies excluded participants who were found after randomisation to have clinical evidence of herpes zoster infection (Lagalla 2002; Taverner 1954). Two studies did not use a standardised scale for assessment of facial motor function (May 1976; Taverner 1954). We considered the effects of these factors on the quality of the evidence insufficient for further downgrading for indirectness of evidence. The results of the majority of studies in this review were consistent with each other, apart from those of one older, smaller study (May 1976).
Potential biases in the review process
To help ensure that decisions about which studies to include in this review were reproducible, two review authors repeated the process (we divided the studies into three groups). There was no distinction made on the experience and expertise of each author in the reviewing pairs. On applying the eligibility criteria and assessing the relevance of studies, review authors were aware of the names of the study authors, institutions, journal of publication and results. FS and FD did not assess their own trial (Sullivan 2007). There were no final disagreements about which studies should be included. According to previous practice in this review, we excluded several studies and a published abstract that provided insufficient information. Therefore, there might be some risk of publication and selective reporting bias due to data from some studies being unavailable.
Agreements and disagreements with other studies or reviews
Our results are coincident with three previous systematic reviews, which found that corticosteroids significantly improved the prognosis of people with Bell's palsy (de Almeida 2009; Ramsey 2000; Williamson 1996). All three reviews, even though of good quality, included a study that lost 50% of participants to follow‐up (Bento 1991). Only one of them, de Almeida 2009, was carried out after the publication of the largest trials included in our review (Engström 2008; Sullivan 2007). Two of them (de Almeida 2009; Ramsey 2000) also included a non‐randomised study in the analyses (Martinez 1990; Shafshak 1994). A Practice Parameter published by the American Academy of Neurology concluded that corticosteroids were safe and probably effective in improving facial functional outcomes in people with Bell's palsy (Grogan 2001). This Practice Parameter was published, also, before the publication of Sullivan 2007 and Engström 2008.
Authors' conclusions
Implications for practice.
According to high‐quality evidence, 10 people with Bell's palsy need to be treated with corticosteroids to avoid one incomplete recovery. The available evidence from randomised controlled trials shows that corticosteroids significantly reduce the frequency of incomplete recovery from Bell's palsy.
Implications for research.
Further research on Bell's palsy treatment should consider giving corticosteroids to those participants acting as controls.
There should be no further studies that do not include corticosteroids as a treatment for Bell's palsy. No participants should be given placebo or no treatment in any future studies. There is an adequate body of evidence to support the use of corticosteroids in the treatment of Bell's palsy.
What's new
| Date | Event | Description |
|---|---|---|
| 4 July 2016 | New citation required but conclusions have not changed | New author team. One new trial was found, with no change to previous conclusions. We made the following corrections and revisions:
|
| 4 March 2016 | New search has been performed | Searches updated and integrated |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 25 February 2009 | New citation required and conclusions have changed | New trials have been added leading to a substantial change in the conclusion of the review |
| 16 July 2008 | Amended | Converted to new review format. |
| 23 June 2008 | New citation required and conclusions have changed | Substantive amendment |
| 28 February 2006 | New search has been performed | February 2006 We updated the search of the Cochrane Neuromuscular Disease Group specialised register (November 2005), MEDLINE (January 1966 to November 2005), EMBASE (January 1980 to November 2005) and LILACS (January 1982 to November 2005). No new randomised controlled trials were found. |
Acknowledgements
We would like to thank the authors of previous editions of this review and we are very grateful for their hard work and enthusiasm. Our thanks are also extended to Angela Gunn who provided the search results and to Cochrane Neuromuscular for their extensive technical assistance and support.
The review has some sections in common with the Cochrane review 'Antiviral treatment for Bell's palsy (idiopathic facial paralysis)' (Gagyor 2015), which has been completed in parallel with this review by the same authors.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Facial Nerve Diseases [REFERENCE] [STANDARD] #2 MeSH DESCRIPTOR Bell Palsy [REFERENCE] [STANDARD] #3 MeSH DESCRIPTOR Facial Paralysis [REFERENCE] [STANDARD] #4 MeSH DESCRIPTOR Hemifacial Spasm [REFERENCE] [STANDARD] #5 (((bell* or facial* or hemifacial* or cranial*) NEAR3 (pals* or paralys* or paresi* or spasm*))) AND (INREGISTER) [REFERENCE] [STANDARD] #6 #1 or #2 or #3 or #4 or #5 [REFERENCE] [STANDARD] #7 MeSH DESCRIPTOR Steroids [REFERENCE] [STANDARD] #8 steroid or steroids [REFERENCE] [STANDARD] #9 MeSH DESCRIPTOR Adrenal Cortex Hormones [REFERENCE] [STANDARD] #10 MeSH DESCRIPTOR Adrenocorticotropic Hormone [REFERENCE] [STANDARD] #11 MeSH DESCRIPTOR Prednisone [REFERENCE] [STANDARD] #12 MeSH DESCRIPTOR Prednisolone [REFERENCE] [STANDARD] #13 MeSH DESCRIPTOR Glucocorticoids [REFERENCE] [STANDARD] #14 MeSH DESCRIPTOR Cortisone [REFERENCE] [STANDARD] #15 corticosteroid* or adrenocorticotroph* or prednisone or prednisolone or glucocorticoid* or cortisone or methylprednisone [REFERENCE] [STANDARD] #16 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 [REFERENCE] [STANDARD] #17 #6 and #16 [REFERENCE] [STANDARD]
Appendix 2. CENTRAL (CRSO) search strategy
Search run on Fri Mar 4 2016 #1 ((Bell or "Bell's" or Bells or facial or hemifacial or cranial) NEAR (palsy or palsies or paralysis or paresis or spasm or spasms)):TI,AB,KY 555 #2 (steroid* or corticosteroid* or adrenocorticotroph* or corticotropin or prednisone or prednisolone or glucocorticoid* or cortisone or methylprednisone or "adrenal cortex hormone*"):TI,AB,KY 37189 #3 (steroid* or corticosteroid* or adrenocorticotroph* or corticotropin or prednisone or prednisolone or glucocorticoid* or cortisone or methylprednisone or "adrenal cortex hormone*"):TI,AB,KY 37189 #4 #1 AND #2 98 #5 sr‐neuromusc:cc 5823 #6 #4 not #5 44
Appendix 3. MEDLINE (OvidSP) strategy
Database: Ovid MEDLINE(R) <1946 to February Week 4 2016> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (407164) 2 controlled clinical trial.pt. (90097) 3 randomized.ab. (304430) 4 placebo.ab. (155333) 5 drug therapy.fs. (1823046) 6 randomly.ab. (215472) 7 trial.ab. (313777) 8 groups.ab. (1364056) 9 or/1‐8 (3457470) 10 exp animals/ not humans.sh. (4191570) 11 9 not 10 (2944190) 12 facial nerve diseases/ (1409) 13 Bell Palsy/ (977) 14 Facial Paralysis/ or hemifacial spasm/ (11784) 15 ((bell$ or facial$ or hemifacial$ or cranial$) adj3 (pals$ or paralys$ or paresi$ or spasm$)).tw. (14759) 16 or/12‐15 (19812) 17 steroid$.tw. or steroids/ (195309) 18 corticosteroid$.tw. or adrenal cortex hormones/ (111371) 19 adrenocorticotroph$.tw. or corticotropin/ (47991) 20 prednisone.tw. or prednisone/ (46046) 21 prednisolone.tw. or prednisolone/ (38861) 22 glucocorticoid$.tw. or glucocorticoids/ (87141) 23 cortisone.tw. or cortisone/ (22428) 24 methylprednisone.tw. (209) 25 or/17‐24 (448308) 26 11 and 16 and 25 (892) 27 remove duplicates from 26 (886)
Appendix 4. EMBASE (OvidSP) strategy
Database: Embase <1980 to 2016 Week 09> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (46158) 2 double‐blind procedure.sh. (126385) 3 single‐blind procedure.sh. (21564) 4 randomized controlled trial.sh. (393423) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1223922) 6 trial.ti. (193286) 7 or/1‐6 (1370827) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1446761) 9 animal/ or nonanimal/ or animal experiment/ (3498115) 10 9 not 8 (2901774) 11 7 not 10 (1261401) 12 limit 11 to embase (1041826) 13 exp facial nerve paralysis/ (20154) 14 Bell palsy/ (2578) 15 hemifacial spasm/ (2209) 16 ((bell$ or facial$ or hemifacial$ or cranial$) adj3 (pals$ or paralys$ or paresi$ or spasm$)).tw. (18906) 17 or/13‐16 (28552) 18 corticosteroid/ or corticosteroid$.tw. (233317) 19 glucocorticoid/ or glucocorticoid$.tw. (101570) 20 corticotropin/ or corticotrophin$.tw. (58083) 21 prednisolone/ or prednisolone.tw. (106302) 22 prednisone/ or prednisone.tw. (142280) 23 or/18‐22 (552861) 24 12 and 17 and 23 (133) 25 remove duplicates from 24 (131)
Appendix 5. LILACS IAHx strategy
("Bell palsy" or "paralisis de Bell" or "paralisia de Bell" or "facial paralysis" or "paralisis facial" or "paralisia facial" or "hemifacial spasm" or "espasmo hemifacial") and (MH:D06.472.040$ or steroids or esteroides or cortisone or cortisona or corticoesteroides or corticosteroides or corticosteroid$ or glucocorticoid$ or prednisolone or prednisolona or prednisone or prednisona or methylprednisone) and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 6. Clinical trials registries search strategies
ClinicalTrials.gov and World Health Organization International Clinical trials Registry
Bell's palsy
Data and analyses
Comparison 1. Corticosteroid (OS) versus placebo or no treatment (OO).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incomplete recovery ≥ 6 months after randomisation | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.50, 0.80] |
| 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.29] |
| 3 Motor synkinesis and crocodile tears | 3 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.91] |
| 4 Adverse effects | 3 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Austin 1993.
| Methods | Double‐blind, randomised, controlled study | |
| Participants | 107 people were initially randomised; 76 completed follow‐up until acute recovery and were included in the study analyses Participants were treated within 5 days of onset Sex: male 39 (51%), female 37 (49%) Age: mean 36.8 years, range 18 to 70 years 37 cases (49%) right side palsy and 39 cases (51%) left side palsy |
|
| Interventions |
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up up to 9 months |
|
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | 1 October 1989 to 31 December 1990 | |
| Notes | Good recovery defined as grade I or II of the House‐Brackmann scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised at the pharmacy but details not given |
| Allocation concealment (selection bias) | Unclear risk | Allocation of participants not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was blinded to both the patient and the clinical investigators". Further details of blinding not given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study was blinded to both the patient and the clinical investigators". Further details of blinding not given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 107 participants randomised, 31 did not attend for follow‐up assessment. 76 allocated to prednisone or placebo Analysis 6 months after resolution of Bell's palsy included 53 participants (23 prednisolone and 30 placebo), representing over 50% loss to follow‐up at this point. Reasons for additional drop‐outs at 6 months not described |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Low risk | Single‐centre study |
Engström 2008.
| Methods | Randomised, placebo‐controlled trial with 4 treatment groups | |
| Participants | 829 participants randomised within 72 hours of facial palsy onset No contraindications to corticosteroid or antiviral use Sex: male 341 (41%), female 488 (59%) Age: mean 40 years, range 31‐54 years |
|
| Interventions | Participants allocated into 1 of 4 treatment groups:
Dosages: valaciclovir 1000 mg 3 times daily for 7 days; prednisolone 60 mg daily for 5 days |
|
| Outcomes | Primary outcome:
Complete recovery was taken as Sunnybrook scale 100 and House‐Brackmann scale grade I Other outcomes:
Follow‐up at 2 weeks, and 1, 2, 3, 6 and 12 months after randomisation, according to recovery Final outcomes reported at 12 months |
|
| Funding | Uppsala University; GlaxoSmithKline (Sweden); Pfizer AB (Sweden); Acta Otolaryngologica Foundation; Rosa and Emanuel Nachmanssons Foundation; Stig and Ragna Gorthon Foundation; Torsten Birger Segerfalk Foundation; Margit Arstrups Foundation; County Council of Skåne; Helsinki University Central Hospital Research Funds | |
| Conflicts of interest | One author was paid by GlaxoSmithKline for a lecture on Bell's Palsy | |
| Date conducted | May 2001 to September 2006 | |
| Notes | Multicentre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by computer number generator. Sequentially numbered identical containers allocated to participants on entry into the trial, by the recruiting physician |
| Allocation concealment (selection bias) | Low risk | Allocation sequence double‐blind and generated by a computer number generator in random permuted blocks of 8 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs issued in identical containers. All participants blinded to treatment group until study completion. All study personnel and data analysts blinded to treatment group until study completion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel and data analysts blinded to treatment group until study completion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers lost to follow‐up and reasons given:
'Modified' intention‐to‐treat analyses used. All randomised participants who received at least 1 dose of study medication included, but participants who did not start therapy excluded. Last observation carried forward method used for the modified intention‐to‐treat analysis, and missing data points imputed in the post‐baseline follow‐up visits from the last observation available for each participant |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. Other outcomes reported in another paper due to space constrictions |
| Other bias | Low risk | No other biases identified |
Lagalla 2002.
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 62 participants within 3 days of onset of onset of Bell's palsy; 4 people excluded after randomisation because of acute herpes zoster infection Sex: male 34, female 28 Age: mean (± SD) 47.5 (± 19) years, range 15 to 84 years Participants with contraindications to corticosteroids (peptic ulcer disease, pregnancy, severe hypertension), or previously treated excluded. No losses to follow‐up 34 cases left palsy, 28 cases right palsy |
|
| Interventions |
All participants received intramuscular vitamins for 15 days |
|
| Outcomes | Primary outcome:
Used House‐Brackmann scale for assessment. "Good recovery" was grades I or II Secondary outcomes:
Follow‐up at 1, 3, 6 and 12 months Final outcomes reported at 6 and 12 months |
|
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | Not stated | |
| Notes | Single centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated by authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to the treatment. Saline solution was used as a placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers lost to follow‐up and reasons given: 62 people randomised, 4 excluded after randomisation (2 in each group) because of herpes zoster infection; the remaining 58 completed study Trial authors stated: "Comparative statistics was carried out on an intention‐to‐treat basis, by assuming that drop‐outs had a poor outcome. Data analysis concerning basal features included all patients enrolled. The analysis of clinical grading changes included data from only 58 subjects" |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported |
| Other bias | Low risk | No other bias identified |
May 1976.
| Methods | Double‐blind, placebo‐controlled, randomised trial with 2 treatment groups | |
| Participants | 51 participants within 2 days of onset of Bell's palsy People with chronic otitis, trauma, loss of lacrimation, and bilateral or recurrent palsy, and herpes zoster excluded Sex: male 28, female 23 Age: ranges not clearly stated "30 years or less and 31 years or older" |
|
| Interventions |
|
|
| Outcomes | Primary outcome:
Assessment made clinically using photographs (examples given in the paper) at 6 months after onset Secondary outcomes:
No adverse effects reported |
|
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | 1972 to 1974 | |
| Notes | Single centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random stratified sequence generated by a statistician, administered in a pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation of treatment group unknown to both participants and physicians |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. Placebo was similar‐looking tablets containing vitamins |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on loss to follow‐up rates |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported, adverse effects not reported |
| Other bias | Low risk | No other bias identified |
Sullivan 2007.
| Methods | Double‐blind, placebo‐controlled, randomised, factorial trial | |
| Participants | 552 participants randomised and 496 included in final outcome assessment Referred for assessment and treatment within 72 hours of paralysis onset. All participants aged ≥ 16 years and no contraindications to corticosteroids or antiviral therapy Sex: male 253 (51%), female 243 (49%) Age: mean (± SD) 44 (± 16.4) years |
|
| Interventions | Participants allocated to 1 of 4 treatment groups to receive:
Participants received prednisolone 25 mg twice daily for 10 days or aciclovir 400 mg 5 times daily for 10 days, both treatments or neither treatment, depending on allocation |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up at 3 and 9 months Final outcomes reported at 9 months |
|
| Funding | Supported by a grant (02/09/04) from the Health Technology Assessment Programme of the National Institute for Health Research (Department of Health, England). The Scottish Executive (Chief Scientist Office and National Health Service Education for Scotland) funded the Scottish School of Primary Care during the study. Practices were reimbursed for their contributions through national Support for Science mechanisms | |
| Conflicts of interest | Drs Sullivan and Donnan reported receiving grant support from GlaxoSmithKline for projects unrelated to the trial. No other potential conflict of interest relevant to this article reported | |
| Date conducted | June 2004 to June 2006 | |
| Notes | Multicentre: 17 hospitals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patient was randomly assigned to a study group by an independent, secure, automated telephone randomisation service" |
| Allocation concealment (selection bias) | Low risk | All parties blinded to allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants not receiving active drug received placebo. All administered medication was identical and in identical containers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Frequency and reason for drop‐outs documented: 138 assigned to prednisolone, of whom 127 completed the trial: 3 received an incorrect drug and 11 were lost to follow‐up (4 withdrew consent, 2 sought active treatment, 1 did not provide primary outcome data, 4 could not be contacted after the 1st visit) 141 assigned to placebo of whom 122 completed the trial: 19 were lost to follow‐up (6 withdrew consent, 3 could not be contacted, 3 sought active treatment, 1 did not provide primary outcome data, 3 could not be contacted after the 1st visit, 2 died, 1 withdrawn by investigator) |
| Selective reporting (reporting bias) | Low risk | All planned outcome measures reported |
| Other bias | Low risk | No other potential sources of bias identified |
Taverner 1954.
| Methods | Double‐blind, randomised controlled trial | |
| Participants | 26 participants within 10 days of onset of Bell's palsy Sex: male 13 and female 13 Age: range 12 to 76 years |
|
| Interventions |
|
|
| Outcomes | Primary outcomes:
Secondary outcome:
Final outcome reported at 157 days |
|
| Funding | Medical Research Council supplied cortisone acetate | |
| Conflicts of interest | Not stated | |
| Date conducted | August 1953 to June 1954 | |
| Notes | Single‐centre study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation centralised in pharmacy, in accordance with a master sheet of random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation in pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Assessors and participants both blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors and participants both blinded (the point to which assessors were blinded was not clear) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 people excluded from analysis because of herpes of the external meatus |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not reported |
| Other bias | Low risk | No other bias identified |
Unuvar 1999.
| Methods | Randomised controlled trial | |
| Participants | 42 children within 3 days of onset of Bell's palsy Sex: male 21, female 21 Age: mean (± SD) 56.9 (± 4.7) months, range 24 to 74 months Children with chronic neurological conditions, other reasons for facial palsy and acute otitis media excluded |
|
| Interventions |
|
|
| Outcomes | Primary outcome:
Secondary outcome:
Follow‐up at 4, 6 and 12 months |
|
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | Not stated | |
| Notes | Single‐centre study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Participants allocated to groups by concealed computer‐generated random sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded to the treatment that they were receiving |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors not blinded to the treatment that participants were receiving |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes given by authors |
| Other bias | Low risk | No other potential biases were identified |
n: number of participants; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akpinar 1979 | Allocation to treatment group was according to the day of admission, which is a method that is highly susceptible to bias. It was not clear who diagnosed participants with Bell's palsy and they used varying dosage regimens of corticosteroids. Follow‐up 3 weeks |
| Bento 1991 | Did not provide the number of participants and outcome events by treatment groups. 50% of participants lost to follow‐up |
| Brown 1982 | Not randomised or quasi‐randomised. There was no placebo group or open control group |
| Martinez 1990 | Not randomised or quasi‐randomised. 27% of participants lost to follow‐up |
| Wolf 1978 | Unable to extract complete information on the specified outcomes |
Characteristics of ongoing studies [ordered by study ID]
Babl 2015.
| Trial name or title | Bell's palsy in children: a multi‐centre, double‐blind, randomised, placebo‐controlled trial to determine whether prednisolone improves recovery at 1 month |
| Methods | Randomised control trial (computer‐generated randomisation schedule), parallel assignment |
| Participants | Participants aged 6 months to 18 years, weight ≥ 5 kg, diagnosed with Bell's palsy by their treating doctor and have an acute onset of symptoms of Bell's palsy for < 72 hours prior to randomisation |
| Interventions |
|
| Outcomes | Primary outcome:
|
| Starting date | 1 July 2015 |
| Contact information | Prof Franz Babl Emergency Research Department, Murdoch Children's Research Institute, Royal Children's Hospital, VIC, Australia +61399366748 franz.babl@rch.org.au |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000563561 |
Differences between protocol and review
A new author team updated this review: Vishnu B Madhok, Ildiko Gagyor, Fergus Daly, Dhruvashree Somasundara, Michael Sullivan, Fiona Gammie, Frank Sullivan.
The review authors added a 'Summary of findings' table and added some sections to the Methods to comply with current Cochrane standards.
Changes from the previous update of this review
When looking at Engström 2008 and Sullivan 2007, we excluded participants that were given antiviral therapy with corticosteroids or antiviral therapy alone as these are reported in a separate review (Gagyor 2015).
As a result, in this update we deleted the comparisons from those trials that the previous review authors designated Sullivan b and Engström b.
We assume that the total number of events given in Engström a (i.e. 213) was incorrect, and changed the figure to 210.
In the previous Cochrane review, authors used intention‐to‐treat numbers. We decided to perform available‐case analysis unless the trialists reported intention‐to‐treat data.
We deleted Sullivan b from Table 1.2 "cosmetically disabling persistent sequelae" for the reasons above. Sullivan a did not contribute data to this table.
In Analysis 1.3, "motor synkinesis of or autonomic dysfunction during follow up", we deleted Engström b for the reasons above.
In Analysis 1.3, we changed the data for Engström a ‐ the previous Cochrane review correctly gave the number of participants reporting synkinesis at 12 months in the OS group as 32. We continued to use this event rate, but changed the total sample number from 290 to 186 in accordance with the information given.
We deleted what was previously Analysis 1.4 "incomplete recovery of patients with Bell's Palsy" as the numbers of participants was negligible (only 22 participants from two studies Sullivan a and May 1976), after we removed the data from Sullivan b.
We checked the numbers for May 1976; Taverner 1954; and Unuvar 1999. We found that the numbers included for Taverner were incorrect and updated these in Analysis 1.1.
The previous Cochrane review excluded Lagalla 2002 from the main meta‐analysis giving the reason that the paper did not provide primary outcome data on complete recovery. We decided to include this study given that we could use information on page 109 (results section) of the paper to calculate the data for input into Analysis 1.1. In the prednisolone group, 32 participants, 83%, had complete recovery, hence 17% (five participants) had incomplete recovery. In the placebo group, there were 30 participants, of whom 25% (eight participants) had incomplete recovery.
Based on our changes to Analysis 1.1, the RR changed from 0.51 (95% CI 0.32 to 0.80) to 0.65 (95% CI 0.50 to 0.83).
We added a table of adverse effects (Analysis 1.4).
1.4. Analysis.
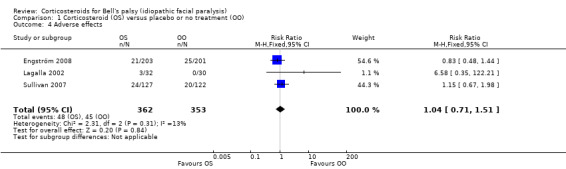
Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 4 Adverse effects.
We checked the data for each of the included studies and made significant amendments and corrections in the Effects of interventions section.
In the Objectives, we deleted the reference to 'early' use of corticosteroids in Bell's palsy.
Contributions of authors
All six review authors worked in pairs on study selection and data extraction.
IG and VM agreed data input into Review Manager 5.
IG performed risk of bias assessments; FS and FD reviewed them.
IG and VM drafted the revised review.
FD assisted with calculations.
VM and IG revised the review after editorial review.
All authors approved the final draft.
Sources of support
Internal sources
New source of support, Other.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Austin 1993 {published data only}
- Austin JR, Peskind SP, Austin SG, Rice DH. Idiopathic facial nerve paralysis: a randomized double blind controlled study of placebo versus prednisone. Laryngoscope 1993;103(12):1326‐33. [PUBMED: 8246650] [DOI] [PubMed] [Google Scholar]
Engström 2008 {published data only}
- Axelsson S, Berg T, Jonsson L, Engström M, Kanerva M, Stjernquist‐Desatnik A. Bell's palsy ‐ the effect of prednisolone and/or valaciclovir versus placebo in relation to baseline severity in a randomised controlled trial. Clinical Otolaryngology 2012;37(4):283‐90. [EMBASE: 2012510516] [DOI] [PubMed] [Google Scholar]
- Berg T, Bylund N, Marsk E, Jonsson L, Kanerva M, Hultcrantz M, et al. The effect of prednisolone on sequelae in Bell's palsy. Archives of Otolaryngology ‐ Head & Neck Surgery 2012;138(5):445‐9. [PUBMED: 22652942] [DOI] [PubMed] [Google Scholar]
- Engström M, Berg T, Stjemquist‐Desatnik A, Axelsson S, Pitkäranta A, Hultcrantz M, et al. Prednisolone and valaciclovir in Bell's palsy: a randomised double‐blind, placebo controlled, multicentre trial. Lancet Neurology 2008;7(11):993‐1000. [PUBMED: 18849193] [DOI] [PubMed] [Google Scholar]
Lagalla 2002 {published data only}
- Lagalla G, Logullo F, Bella P, Provinciali F, Ceravolo MG. Influence of early high‐dose steroid treatment on Bell's palsy evolution. Neurological Sciences 2002;23(3):107‐12. [PUBMED: 12391494] [DOI] [PubMed] [Google Scholar]
May 1976 {published data only}
- May M, Wette R, Hardin WB Jr, Sullivan J. The use of steroids in Bell's palsy: a prospective controlled study. Laryngoscope 1976;86(8):1111‐22. [PUBMED: 781439] [DOI] [PubMed] [Google Scholar]
Sullivan 2007 {published and unpublished data}
- Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al. Early treatment with prednisolone or acyclovir in Bell´s Palsy. New England Journal of Medicine 2007;357(16):1598‐607. [PUBMED: 17942873] [DOI] [PubMed] [Google Scholar]
Taverner 1954 {published data only}
- Taverner D. Cortisone treatment of Bell's palsy. Lancet 1954;264(6847):1052‐4. [PUBMED: 13213115] [DOI] [PubMed] [Google Scholar]
Unuvar 1999 {published data only}
- Unuvar E, Oguz F, Sidal M, Kilic A. Corticosteroid treatment of childhood Bell's palsy. Pediatric Neurology 1999;21(5):814‐6. [PUBMED: 10593672] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akpinar 1979 {published data only}
- Akpinar S, Boga M, Yardim M. Steroid versus placebo treatments in cases of acute peripheral facial paralysis [Akut periferik fasiyel paralizi olgularinda steroid tedavisinin plasebo ile oranlanmasi]. Bulletin of Gulhane Military Medical Academy 1979;21:45‐51. [Google Scholar]
Bento 1991 {published data only}
- Bento RF, Bogar P, Lorenzi MC. Treatment comparison between dexamethasone and placebo for idiopathic facial palsy. European Archives of Oto‐Rhino‐Laryngology 1994;suppl:S535‐6. [PUBMED: 10774443] [DOI] [PubMed] [Google Scholar]
- Bento RF, Lorenzi MC, Bogar P, Marone SA, Minili A. Comparison between dexametasone and placebo for idiopathic facial palsy [Comparacao entre a dexametasona e placebo no tratamento da paralisia facial periferica idiopatica]. Revista Brasileira de Otorrinolaringologia 1991;57:196‐202. [EMBASE: 92038773] [Google Scholar]
Brown 1982 {published data only}
- Brown JS. Bell's palsy: a 5 year review of 174 consecutive cases: an attempted double blind study. Laryngoscope 1982;92(12):1369‐73. [PUBMED: 6757616] [DOI] [PubMed] [Google Scholar]
Martinez 1990 {published data only}
- Martinez CG, Abarca B, Alvardo CL. Bell's palsy: evaluation of steroid treatment [Paralisis de Bell: Evaluacion del tratamiento esteroidal]. Boletin del hospital de San Juan de Dios 1990;37(1):13‐7. [Google Scholar]
Wolf 1978 {published data only}
- Wolf SM, Wagner JH, Davidson S, Forsythe A. Treatment of Bell palsy with prednisone: a prospective, randomized study. Neurology 1978;28(2):158‐61. [PUBMED: 340980] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Babl 2015 {unpublished data only}
- Bell's palsy in children: a multi‐centre, double‐blind, randomised, placebo‐controlled trial to determine whether prednisolone improves recovery at 1 month. Ongoing study 1 July 2015.
Additional references
Adour 1972
- Adour KK, Wingerd J, Bell DN, Manning JJ, Hurley JP. Prednisone treatment for idiopathic facial paralysis (Bell's palsy). New England Journal of Medicine 1972;287(25):1268‐72. [DOI] [PubMed] [Google Scholar]
Adour 1982
- Adour KK. Current concepts in neurology: diagnosis and management of facial paralysis. New England Journal of Medicine 1982;307(6):348‐51. [DOI] [PubMed] [Google Scholar]
Bateman 1992
- Bateman JE. Facial palsy. British Journal of Hospital Medicine 1992;47(6):430‐31. [PubMed] [Google Scholar]
Brandenberg 1993
- Brandenberg NA, Annegers JF. Incidence and risk factors for Bell's palsy in Laredo, Texas. Neuroepidemiology 1993;12(6):313‐25. [DOI] [PubMed] [Google Scholar]
Burgess 1984
- Burgess LP, Yim DW, Lepore ML. Bell's palsy: the controversy revisited. Laryngoscope 1984;94(11 pt 1):1472‐6. [PubMed] [Google Scholar]
de Almeida 2009
- Almeida JR, Al Khabori M, Guyatt GH, Witterick IJ, Lin VYW, Nedzelski JM, et al. Combined corticosteroid and antiviral treatment for Bell palsy: a systematic review and meta‐analysis. JAMA 2009;302(9):985‐93. [DOI] [PubMed] [Google Scholar]
Dollery 1999
- Dollery C. Therapeutic Drugs. 2nd Edition. Vol. 2, Edinburgh: Churchill Livingstone, 1999. [ISBN: 0443051488 (2 volumes)] [Google Scholar]
Egger 2007
- Egger M, Davey Smith G, Altman DG. Systematic Reviews in Health Care: Meta‐Analysis in context. 6th Edition. London: BMJ Books, 2007. [Google Scholar]
Gagyor 2015
- Gagyor I, Madhok VB, Daly F, Somasundara D, Sullivan M, Gammie F, et al. Antiviral treatment for Bell's palsy (idiopathic facial paralysis). Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD001869.pub8] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- McMaster University. GRADEpro. Version 3.2 for Windows. McMaster University, 2008.
Grogan 2001
- Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell's palsy (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56(7):830‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katusic 1986
- Katusic SK, Beard CM, Wiederholt WC, Bergstrahl EJ, Kurland LT. Incidence, clinical features, and prognosis in Bell's palsy, Rochester, Minnesota. Annals of Neurology 1986;20(5):622‐7. [DOI] [PubMed] [Google Scholar]
Lackner 2010
- Lackner A, Kessler HH, Walch C, Quasthoff S, Raggam RB. Early and reliable detection of herpes simplex virus type 1 and varicella zoster virus DNAs in oral fluid of patients with idiopathic peripheral facial nerve palsy: decision support regarding antiviral treatment?. Journal of Medical Virology 2010;82(9):1582‐5. [DOI] [PubMed] [Google Scholar]
Madhok 2009
- Madhok V, Falk G, Fahey T, Sullivan F. Prescribe prednisolone alone for Bell's palsy diagnosed within 72 hours of symptom onset. BMJ 2009;338:255. [DOI] [PubMed] [Google Scholar]
Martyn 1997
- Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. Journal of Neurology, Neurosurgery & Psychiatry 1997;62(4):310‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morales 2013
- Morales DR, Donnan PT, Daly F, Staa T, Sullivan F. Impact of clinical trial findings on Bell's palsy management in general practice in the UK 2001‐2012: interrupted time series regression analysis. BMJ Open 2013;3(7):e003121. [DOI: 10.1136/bmjopen-2013-003121; PUBMED: 23864211] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murakami 1996
- Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Annals of Internal Medicine 1996;124(1 pt 1):27‐30. [DOI] [PubMed] [Google Scholar]
Niparko 1993
- Niparko JK, Mattox DE. Bell's palsy and herpes zoster oticus. In: Johnson RT, Griffin JW editor(s). Current Therapy in Neurologic Disease. 4th Edition. St. Louis, MO: BC Decker, 1993:355‐61. [ISBN: 0801677300] [Google Scholar]
Peitersen 1982
- Peitersen E. The natural history of Bell's palsy. American Journal of Otology 1982;4(2):107‐11. [PubMed] [Google Scholar]
Peitersen 2002
- Peitersen E. Bell's palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngologica Supplementum 2002;549:4‐30. [PubMed] [Google Scholar]
Prescott 1988
- Prescott CA. Idiopathic facial nerve palsy (the effect of treatment with steroids). Journal of Laryngology & Otology 1988;102(5):403‐7. [DOI] [PubMed] [Google Scholar]
Ramsey 2000
- Ramsey MJ, DerSimonian R, Holtel MR, Burgess LP. Corticosteroid treatment for idiopathic facial nerve palsy: a meta analysis. Laryngoscope 2000;110(3 pt 1):335‐41. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shafshak 1994
- Shafshak TS, Essa AY, Bakey FA. The possible contributing factors for the success of steroid therapy in Bell's palsy: a clinical and electrophysiological study. Journal of Laryngology and Otology 1994;108(11):940‐3. [DOI] [PubMed] [Google Scholar]
Victor 1994
- Victor M, Martin J. Disorders of the cranial nerves. In: Isselbacher KJ, Wilson J, Fauci A, Braunwald E, Martin J, Kasper D editor(s). Harrison's Principles of Internal Medicine. 13th Edition. New York: McGraw‐Hill, 1994:2347‐52. [ISBN: 0070323704] [Google Scholar]
Williamson 1996
- Williamson IG, Whelan TR. The clinical problem of Bell's palsy: is treatment with steroids effective?. British Journal of General Practice 1996;46(413):743‐7. [PMC free article] [PubMed] [Google Scholar]
Yanagihara 1988
- Yanagihara N, Yumoto E, Shibahara T. Familial Bell's palsy: analysis of 25 families. Annals of Otology, Rhinology and Laryngology 1988;137(Suppl):8‐10. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Salinas 2002
- Salinas RA, Alvarez G, Alvarez MI, Ferreira J. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001942] [DOI] [PubMed] [Google Scholar]
Salinas 2004
- Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001942.pub2] [DOI] [PubMed] [Google Scholar]
Salinas 2009
- Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001942.pub3] [DOI] [PubMed] [Google Scholar]
Salinas 2010
- Salinas RA, Alvarez G, Daly F, Ferreira J. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD001942.pub4] [DOI] [PubMed] [Google Scholar]


