Abstract
Background
Chagas disease‐related cardiomyopathy is a major cause of morbidity and mortality in Latin America. Despite the substantial burden to the healthcare system, there is uncertainty regarding the efficacy and safety of pharmacological interventions for treating heart failure in people with Chagas disease. This is an update of a Cochrane review published in 2012.
Objectives
To assess the clinical benefits and harms of current pharmacological interventions for treating heart failure in people with Chagas cardiomyopathy.
Search methods
We updated the searches in the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 1), MEDLINE (Ovid; 1946 to to February Week 1 2016), EMBASE (Ovid; 1947 to 2016 Week 07), LILACS (1982 to 15 February 2016), and Web of Science (Thomson Reuters; 1970 to 15 February 2016). We checked the reference lists of included papers. We applied no language restrictions.
Selection criteria
We included randomised clinical trials (RCTs) that assessed the effects of pharmacological interventions to treat heart failure in adult patients (18 years or older) with symptomatic heart failure (New York Heart Association classes II to IV), regardless of the left ventricular ejection fraction stage (reduced or preserved), with Chagas cardiomyopathy. We did not apply limits to the length of follow‐up. Primary outcomes were all‐cause mortality, cardiovascular mortality at 30 days, time‐to‐heart decompensation, disease‐free period (at 30, 60, and 90 days), and adverse events.
Data collection and analysis
Two authors independently performed study selection, 'Risk of bias' assessment and data extraction. We estimated relative risk (RR) and 95% confidence intervals (CIs) for dichotomous outcomes. We measured statistical heterogeneity using the I² statistic. We used a fixed‐effect model to synthesize the findings. We contacted authors for additional data. We developed 'Summary of findings' (SoF) tables and used GRADE methodology to assess the quality of the evidence.
Main results
In this update, we identified one new trial. Therefore, this version includes three trials (108 participants). Two trials compared carvedilol against placebo and another assessed rosuvastatin versus placebo. All trials had a high risk of bias.
Meta‐analysis of two trials showed a lower proportion of all‐cause mortality in the carvedilol groups compared with the placebo groups (RR 0.69; 95% CI 0.12 to 3.88, I² = 0%; 69 participants; very low‐quality evidence). Neither of the trials reported on cardiovascular mortality, time‐to‐heart decompensation, or disease‐free periods.
One trial (30 participants) found no difference in hospital readmissions (RR 1.00; 95% CI 0.31 to 3.28; very low‐quality of evidence) or reported adverse events (RR 0.92; 95% CI 0.67 to 1.27; very low‐quality of evidence) between the carvedilol and placebo groups.
There was very low‐quality evidence from two trials of inconclusive effects on quality of life (QoL) between the carvedilol and placebo groups. One trial (30 participants) assessed QoL with the Minnesota Living With Heart Failure Questionnaire (21 items; item scores range from 0 to 5; a lower MLHFQ score is better). The MD was ‐14.74; 95% CI ‐24.75 to ‐4.73. The other trial (39 participants) measured QoL with the Medical Outcomes Study 36‐item short‐form health survey (SF‐36; item scores range from 0 to 100; higher SF‐36 score is better). Data were not provided.
One trial (39 participants) assessed the effect of rosuvastatin versus placebo. The trial did not report on any primary outcomes or adverse events. There was very low‐quality evidence of uncertain effects on QoL (no data were provided).
Authors' conclusions
This first update of our review found very low‐quality evidence for the effects of either carvedilol or rosuvastatin, compared with placebo, for treating heart failure in people with Chagas disease. The three included trials were underpowered and had a high risk of bias. There were no conclusive data to support or reject the use of either carvedilol or rosuvastatin for treating Chagas cardiomyopathy. Unless randomised clinical trials provide evidence of a treatment effect, and the trade‐off between potential benefits and harms is established, policy‐makers, clinicians, and academics should be cautious when recommending or administering either carvedilol or rosuvastatin to treat heart failure in people with Chagas disease. The efficacy and safety of other pharmacological interventions for treating heart failure in people with Chagas disease remains unknown.
Plain language summary
Pharmacological interventions for treating heart failure in patients with Chagas cardiomyopathy
Review question We reviewed pharmacological interventions for treating heart failure in people with Chagas cardiomyopathy.
Background Named in honour of the Brazilian physician Carlos Chagas, Chagas disease is caused by the Trypanosoma cruzi parasite. It is common in Latin and Central America and leads to Chagas cardiomyopathy (heart muscle disease). It is an important cause of heart failure. The number of people infected with Chagas disease has been estimated to be about 10 to 12 million worldwide; around 20% to 30% of individuals infected with Trypanosoma cruzi will develop symptomatic heart disease at some point during their lives. In the Americas in 2005, there were estimated to be 7,694,500 people infected by Trypanosoma cruzi and 1,772,365 suffering from chagasic cardiomyopathy. Infected people from endemic countries in Latin America are migrating throughout the world. As a result, what was thought to be a health problem in the Americas is rapidly becoming a world health problem. It has been estimated that 300,167 individuals with Trypanosoma cruzi infection live in the United States, with 30,000 to 45,000 cardiomyopathy cases and 63 to 315 congenital infections annually. Standard treatment options for non‐Chagas disease heart failure are used for treating Chagas disease‐related heart failure. However, because of fundamental differences in the affected populations, it is important to assess the benefits and harms of pharmacological interventions for Chagas disease‐related heart failure.
Study characteristics We identified one new trial, so there are now three studies involving 108 participants. All studies were conducted in Brazil during 2004, 2007, and 2012. Two trials evaluated the effects of carvedilol versus placebo; one trial assessed rosuvastatin versus placebo.
Key results The results were inconclusive that carvedilol reduced all‐cause mortality or improved quality of life more than placebo. The safety profile of carvedilol for Chagas cardiomyopathy remains unclear. One study assessed the effect of rosuvastatin versus placebo, but did not show an effect size. Therefore, the results from available clinical trials neither support nor reject the use of carvedilol or rosuvastatin in treating this clinical entity. Further investigation is warranted to investigate the exact applicability of conventional heart failure treatment agents in Chagas cardiomyopathy.
Quality of evidence Our confidence in the results of this review is very low because the included trials had a high risk of bias and were small. which generated imprecise results.
Date searched: 15 February 2016.
Summary of findings
Background
Description of the condition
Definition and epidemiology
The pathogen and clinical manifestations of Chagas disease, named after Carlos Chagas, a Brazilian physician, were first discovered in 1909 (Labarthe 1998; Moncayo 2010). Chagas disease is also known as human American trypanosomiasis, and is endemic in the American continent (Moncayo 2006; Moncayo 2009). It is caused by the parasite Trypanosoma cruzi (T. cruzi) and is the major cause of infectious myocarditis worldwide (Andrade 2011; Figure 1).
1.
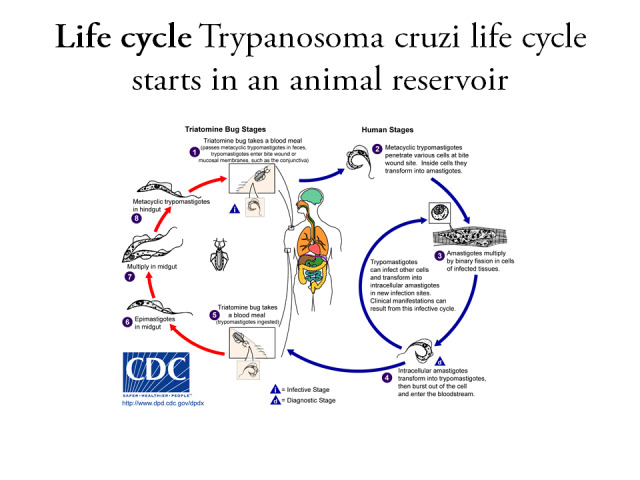
Trypanozoma cruzi life cycle. Reproduced with permission from CDC.
One of the clinical forms of Chagas disease is "Chagas disease (chronic) with heart involvement" (Labarthe 1998). Chagas disease is still a major cause of heart failure in South America (Khatibzadeh 2013; Mendez 2001; Malik 2015a) and thus remains an important health problem (Rassi 2006). The number of people infected with Chagas disease has been estimated to be about 10 to 12 million worldwide, and it is estimated that 20% to 30% of individuals infected with T. cruzi will develop symptomatic heart disease at some point during their lives (Gascón 2007). Furthermore, there are an estimated 200,000 new cases per year in 15 Latin American countries (Costa 2012). Table 3 shows the burden of the infected population in America, where in 2005, there were 7,694,500 people infected by Trypanosoma cruzi and 1,772,365 suffering from Chagasic cardiopathy (OPS 2006). Table 4 shows the epidemiology of infected people from endemic countries in Latin America migrating throughout the world. This shows how an issue that was considered an American health problem is rapidly becoming a world health problem (Schmunis 2010). Bern 2009 has estimated that 300,167 individuals with T. cruzi infection live in the United States. With 30,000 to 45,000 cardiomyopathy cases and 63 to 315 congenital infections annually, T.cruzi causes a substantial burden of disease in the United States (Bern 2009; Malik 2015b; Melton 2015; Traina 2015). Preventive approaches, such as control of the Triatomine bug and ecological niche studies, are key to reducing the incidence of Chagas disease (Carrasco 2012; Cruz‐Pacheco 2012; Gurgel‐Goncalves 2012; Yamagata 2006). The economic burden of Chagas disease could be higher than diseases such as, rotavirus, cervical cancer, and Lyme disease (Lee 2013).
1. Burden of infected population in the Americas and by region.
|
Variable (2005) |
The Americas | Southern Cone | Andean Community | Centroamerican region and Belize | French Guayana, Guyana, Suriname | Mexico | USA |
| Population | 531,432,850 | 259,805,650 | 113,545,000 | 39,656,200 | 1,397,000 | 107,029,000 | ‐ |
| Infected | 7,694,500 | 4,451,900 | 1,168,000 | 806,600 | 18,000 | 1,100,000 | 100,000 to 200,000 people from endemic countries. |
| Congenital Chagas (annual) | 14,385 | 9,365 | 2,600 | 1,300 | 20 | 1,100 | ‐ |
| Chagas cardiopathy | 1,772,365 | 1,180,990 | 361,954 | 129,345 | 933 | 99,143 | ‐ |
Data from OPS 2006.
2. Epidemiology of infected immigrants from Latin America endemic countries to the world.
| Destination country | Year | Infected Immigrants from Latin American endemic countries | Immigrants with chronic Chagas disease |
| Australia | 2006 | 3.8% of 80,522 | Not described. |
| Canada | 2006 | 3.5% of 156,960 | Not described. |
| Japan | 2007 | 80,912 immigrants from Brazil, 15,281 from Peru, and 19,413 from other South American countries whose country of origin was not identified. Information about infected people was not supplied. |
Not described. |
| Europe (15 countries excluding Spain) | 2005 | 2.9% of 483,074 legal Latin American immigrants. | Not described. |
| Spain | 2007 | 5.2% of 1,678,711. 24 to 92 newborns born to South American T. cruzi infected mothers in Spain may have been congenitally infected with T. cruzi in 2007 | 17,390 |
| USA | 2000 2007 | 1.9% of approximately 13 million Latin American immigrants. 2% of 17 million. | 49,157 65,133 |
Data from Schmunis 2010.
Etiology of Chagas disease
Chagas disease is an acquired inflammatory cardiomyopathy characterized by chronic fibrosing myocarditis (varying from focal or multifocal to diffuse; Rassi Jr 2009; Rossi 1991). The etiology of Chagas disease is multifactorial (Marin‐Neto 2007). Parasite persistence has been hypothesized as a cause (Dávila 2002b; Zhang 1999); however, controversy exists about it (Elias 2003). Autoimmunity is another pathogenic mechanism (Dávila 2002b; Tanowitz 2009). Chagas disease has been considered to be a paradigm of infection‐induced autoimmune disease (Gironès 2005; Gironès 2007). Autoimmune reactions seem to be mediated by a T. cruzi protein, Trypanosoma cruzi calreticulin (Ribeiro 2009). The role of autoantibodies in the physiopathology of Chagas disease has been described (Medei 2008). Recently, the immunopathology and genetics aspects of Chagas disease cardiomyopathy were extensively reviewed, and it was concluded that Th1 t‐cell‐rich myocarditis, with cardiomyocyte hypertrophy and prominent fibrosis are prominent findings in this disease (Cunha‐Neto 2014). There is strong evidence that it develops as a result of additive and even synergistic effects of several distinct mechanisms, rather than from one factor (Bonney 2008).
The pathogenesis of Chagas disease is not completely understood, but the evidence suggests that it could be explained by four pathogenetic mechanisms: direct parasite damage to the myocardium, immunologic mechanisms, dysautonomia, and microvascular disturbances (Biolo 2010; Dávila 2004; Dávila 2005). The complexity of the immune response generated during T. cruzi infection strengthens the concept that the host immune response is critical for disease control and evolution (Dutra 2008; Esper 2015).
Pathophysiology and cardiovascular clinical manifestations
Pathophysiology of Chagas disease has been reviewed widely by Rassi Jr 2009 and Higuchi 2003. The cardiac clinical form is caused by an inflammatory reaction in the heart tissue, leading to a spectrum of debilitating and morbid cardiac diseases (Dutra 2008). The diagnostic triad suggestive of Chagas disease includes: a) epidemiological history; b) positive serology (antibodies against T. cruzi) in at least two tests; and c) clinical findings such as: heart failure; syncope; complex arrhythmias; embolisms; electrocardiographic findings, such as right bundle block, left anterior hemiblock, or a combination of the latter two conditions; ventricular extrasystoles; ST‐T segment anomalies; and apical aneurysm of the left ventricle, among others (Acquatella 2008; Machado 2012; Ribeiro 2012b). These syndromes are caused by inflammatory lesions and an immune response, particularly mediated by either CD4‐positive T‐lymphocytes, CD8‐positive T‐lymphocytes, interleukin‐2 or interleukin‐4 with cell and neuron destruction and fibrosis (Coura 2010). Congestive heart failure is more commonly expressed by prominent signs of systemic congestion, with less intense pulmonary congestion. This peculiar feature of Chagas disease is linked to early severe damage of the right ventricle, a chamber frequently neglected in investigations of cardiac function (Marin‐Neto 1998). Patients with congestive heart failure secondary to Chagas cardiomyopathy have a poorer prognosis than those with congestive heart failure secondary to hypertension (Bestetti 2013), which could be explained by malignant ventricular arrhythmias, which lead to sudden cardiac death (Veloso 2014). However, it is controversial (Betestti 2014).
In the acute phase, death is mostly caused by myocarditis, and in the chronic phase, by irreversible cardiomyopathy (Punukollu 2007). It has been suggested that inflammatory cardiomyopathy of Chagas' disease is a genetically driven autoimmune disease (Teixeira 2011).
Mortality during the acute phase of cardiac Chagas is around 5%, while five‐year mortality of chronic Chagas disease with cardiac dysfunction is above 50% (Punukollu 2007). Pathological findings in the heart include mononuclear inflammatory infiltrate, focal myocarditis, epicarditis and neuroganglionitis, associated with variable focal fibrosis and widely variable autonomic dysfunction (Ribeiro 2012a). The immune‐inflammatory response has been considered to be the cause of the autonomic dysfunction, which may trigger life‐threatening arrhythmias and sudden death (Ribeiro 2012a).
The risk of mortality in patients affected by Chagas disease includes three stages: low (total mortality: 2% and 10% at five years and 10 years, respectively), intermediate (total mortality: 18% and 44% at five years and 10 years, respectively), and high (total mortality: 63% and 84% at five years and 10 years, respectively; Rassi Jr 2010). This stratification of risk of death has led to the following recommended approaches, which are based on expert opinion rather than evidence of benefit (Rassi Jr 2010):
Low stage without New York Heart Association (NYHA) class III or IV, left ventricular systolic dysfunction (echocardiography), cardiomegaly (chest radiography), or both, and non‐sustained ventricular tachycardia (24‐hour Holter monitoring) ‐ possibly treat with antiparasitic drug.
Intermediate stage without NYHA class III or IV, left ventricular systolic dysfunction (echocardiography), cardiomegaly (chest radiography), or both, but with non‐sustained ventricular tachycardia (24‐hour Holter monitoring) ‐ possibly treat with amiodarone and an antiparasitic drug.
Intermediate stage without NYHA class III or IV, with left ventricular systolic dysfunction (echocardiography), cardiomegaly (chest radiography), or both, but absence of non‐sustained ventricular tachycardia (24‐hour Holter monitoring) ‐ treat with angiotensin‐converting enzyme inhibitors, beta‐blockers, diuretics (for selected patients), and possibly treat with an antiparasitic drug.
High stage without NYHA class III or IV, with left ventricular systolic dysfunction (echocardiography), cardiomegaly (chest radiography), or both, and with non‐sustained ventricular tachycardia (24‐h Holter monitoring) ‐ treat with angiotensin‐converting enzyme inhibitors, amiodarone, diuretics (for selected patients), beta‐blockers (if clinically tolerated), and possibly treat with an implantable cardioverter defibrillator.
High stage with NYHA class III or IV, with left ventricular systolic dysfunction (echocardiography), cardiomegaly (chest radiography), or both, and with non‐sustained ventricular tachycardia (24‐hour Holter monitoring) ‐ treat with angiotensin‐converting enzyme inhibitors, spironolactone, amiodarone, diuretics, digitalis, beta‐blockers (if clinically tolerated), heart transplantation (if clinically tolerated), and possibly treat with an implantable cardioverter defibrillator.
Description of the intervention
In Chagas disease, the haemodynamic and neurohormonal responses are similar to those in other cardiomyopathies. This common pathophysiology suggests that therapies effective in usual heart failure cases should also be beneficial in Chagas disease (Botoni 2007). Pharmacological agents such as angiotensin‐converting enzyme inhibitors and beta‐blockers are likely to be as important in Chagas disease as in other heart failure syndromes (Biolo 2010). Serious adverse events have been observed with these medications in chronic heart failure. See Appendix 1 for adverse events from pharmacological therapy to treat heart failure.
Pharmacological interventions for treating heart failure include many different families of drugs (Adorisio 2006; Hamad 2007; Mills 2001):
Angiotensin‐converting enzyme inhibitors: captopril, lisinopril, fosinopril sodium, enalapril maleate, benazepril, quinapril, ramipril;
Angiotensin II receptor antagonists: losartan, candesartan, valsartan, irbesartan;
Aldosterone receptor antagonists: spironolactone, eplerenone;
Inotropes: milrinone, dobutamine;
Digitalis: digoxin;
Diuretics: furosemide;
Vasodilators: isosorbide dinitrate, hydralazine, nitroprusside, nesiritide (recombinant human B‐type natriuretic peptide);
Beta‐adrenoceptor antagonists: carvedilol, metoprolol, bisoprolol;
Calcium sensitizers: pimobendan, levosimendan.
There is insufficient evidence to support the efficacy of nitrofurans or imidazolic drugs for treating overt Chagas disease (Reyes 2005). The existing evidence on its prevention indicates a need to test these and newer agents in more and larger RCTs that include clinical outcomes for chronic asymptomaticT. cruzi infection (Villar 2002).
Trypanocidal efficacy of posaconazole and ravuconazole is being tested (Buckner 2010; Diniz 2010; Olivieri 2010). Recently, the relevance and current limitations of, and new approaches to, specific chemotherapy for Chagas disease have been reviewed (Urbina 2010).
How the intervention might work
The above‐mentioned pharmacological interventions work through many different mechanisms (Hamad 2007).
Angiotensin‐converting enzyme inhibitors reduce angiotensin II production by blocking the plasma and pulmonary endothelial angiotensin‐converting enzyme. Angiotensin II produces deleterious cardiovascular effects including direct vasoconstriction, increased sympathetic discharge, release of catecholamines, increased sodium reabsorption in the proximal tubule, and the release of aldosterone.
Angiotensin II receptor antagonists block the effects of the angiotensin II, which generates the activation of two types of receptors on the cell surface: angiotensin II type 1 and angiotensin II type 2. Angiotensin II receptor antagonists type1 mediate vasoconstriction and stimulate aldosterone and vasopressin secretion, which cause sodium and water retention.
Aldosterone receptor antagonists reduce the action of aldosterone, a hormone produced by the adrenal glands. Aldosterone causes vasoconstriction, increases salt and water retention, and stimulates the growth of fibroblasts and the synthesis of collagen.
Inotropes cause increased inotropic effects and vasodilation independent of the stimulation of beta‐receptors (milrinone), or through the stimulation of the of beta‐receptors of the heart.
Digitalis leads to increased myocardial contractility through the increase of intracellular calcium.
Diuretics increase the excretion of sodium and water, which reduces fluid retention.
Vasodilators reduce afterload and preload by dilating both arterial and venous blood vessels.
Beta‐adrenoceptor antagonists reduce the sympathetic nervous system and renin‐angiotensin system.
Calcium sensitisers increase myocardial contractility.
Why it is important to do this review
A review of the evidence for treating heart failure associated with Chagas disease is required for the following reasons:
First, Chagas disease is a major cause of morbidity and mortality in Latin America (Rassi 2000; Schmunis 2010). Second, treatment of Chagas' cardiomyopathy during acute decompensated heart failure is very expensive (Abuhab 2013). Third, although there are published systematic reviews of the effect of trypanocidal drugs for the different stages of Chagas disease, no systematic review of the pharmacological interventions commonly used in chronic heart failure has been conducted for Chagas disease (Reyes 2005; Villar 2002). Fourth, the management of Chagas disease may be even more difficult than that of other dilated cardiomyopathies (Dobarro 2008). This worse prognosis may be due to a greater degree of cardiac impairment (lower ejection fraction) and haemodynamic instability (lower systolic blood pressure and heart rate), increased activation of the renin‐angiotensin system, and increased cytokine levels (Silva 2008), and reduces the quality of life (Sousa 2015). Therefore, there are uncertainties about the benefits of using pharmacological interventions, and the rates of their adverse effects. Drugs for treating heart failure are associated with severe adverse events, which in patients with Chagas disease, could be life‐threatening. Fifth, the increasing number of people affected by Chagas disease emigrating from the Americas to developed countries may cause a radical increase in the incidence of this disease over the coming years, however, European cardiologists are unfamiliar with this chronic cardiomyopathy (Bimbi 2014; Dobarro 2008; Gascón 2010; Gascón 2007; Muñoz 2009; Soriano 2009; Strasen 2014; Table 4). Therefore, a review is needed to improve patient care through therapeutic decision making, based on the best evidence‐based treatment.
This is an update of a Cochrane review previously published in 2012, which sought to answer the research question: "What are the benefits and harms of pharmacological interventions for treating heart failure in patients with Chagas cardiomyopathy?" (Hidalgo 2012).
Appendix 2 provides a medical glossary.
Objectives
To assess the clinical benefits and harms of current pharmacological interventions for treating heart failure in people with Chagas cardiomyopathy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials, regardless of publication status (trials may be unpublished or published as an article, an abstract, or a letter). We applied no language, country, or sample size limitations. We included trials conducted in either a hospital or community setting, or both. We applied no limits on the length of follow‐up.
Types of participants
Adults (18 years or older) with symptomatic heart failure (New York Heart Association class II to IV; Table 5), regardless of whether the left ventricular ejection fraction stage was reduced or preserved, in patients with Chagas cardiomyopathy. We considered trials that evaluated pharmacotherapies in a general heart failure population that included participants affected by Chagas cardiomyopathy.
3. New York Heart Association (NYHA) Classification System.
| NYHA class I (mild) | NYHA class II (mild) | NYHA class III (moderate) | NYHA class IV (severe) |
| No limitation of physical activity ‐ ordinary physical activity does not cause tiredness, heart palpitations, or shortness of breath. | Slight limitation of physical activity ‐ comfortable at rest, but ordinary physical activity results in tiredness, heart palpitations, or shortness of breath. | Marked or noticeable limitations of physical activity ‐ comfortable at rest, but less than ordinary physical activity causes tiredness, heart palpitations, or shortness of breath. | Severe limitation of physical activity ‐ unable to carry out any physical activity without discomfort. Symptoms also present at rest. If any physical activity is undertaken, discomfort increases. |
Types of interventions
Interventions
Angiotensin converting enzyme inhibitors (ACE inhibitors): captopril, lisinopril, fosinopril sodium, enalapril maleate, benazepril, quinapril, ramipril;
Angiotensin II receptor antagonists: losartan, candesartan, valsartan, irbesartan;
Aldosterone receptor antagonists: spironolactone, eplerenone;
Inotropes: milrinone, dobutamine;
Digitalis: digoxin;
Diuretics: furosemide;
Vasodilators: isosorbide dinitrate, hydralazine, nitroprusside, nesiritide (recombinant human B‐type natriuretic peptide);
Beta‐adrenoceptor antagonists: carvedilol, metoprolol, bisoprolol;
Calcium sensitisers: pimobendan, levosimendan.
Comparisons
Placebo;
Standard care (low‐salt diet, rest);
Any head‐to‐head comparisons.
Types of outcome measures
Primary outcomes
All‐cause mortality;
Cardiac mortality at 30 days;
Time‐to‐heart decompensation;
Disease‐free period (at 30, 60, and 90 days).
Secondary outcomes
Overall survival, defined as "the proportion of persons in a specified group, alive at the beginning of the time interval, who survive to the end of the interval" (Porta 2008);
Quality of life, measured with any validated scale;
Hospital readmissions (heart failure‐ or adverse event‐related);
Adherence grade, which will be measured as the proportion of time patients took more than 80% of study medication (Granger 2009);
Adverse events, classified as "any untoward medical occurrence that may present during treatment with a pharmaceutical product, but which does not necessarily have a causal relationship with this treatment" (Nebeker 2004);
Digoxin toxicity: extra‐cardiac, or cardiac, or both, signs and symptoms attributed to digoxin. These clinical manifestation are more common above 2.5 nmol/L (2.0 μg/L). Extra‐cardiac manifestation include visual disturbances, anorexia, nausea, or vomiting. Cardiac manifestations include rhythm disturbances (Bauman 2006).
Search methods for identification of studies
Electronic searches
We updated the searches run in 2011 (Appendix 3), on 16 February 2016 (Appendix 4).
The following databases were searched:
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2016, Issue 1),
MEDLINE (Ovid; 1946 to February Week 1 2016),
EMBASE (Ovid; 1947 to 2016 Week 07),
LILACS (1986 to 15 February 2016), and
Web of Science (Thomson Reuters, 1970 to 15 February 2016).
We used the Cochrane sensitive‐maximising RCT filters to search MEDLINE and EMBASE (Lefebvre 2011).
We imposed no language restrictions.
Searching other resources
We updated the searches of the Clinical Trials Search Portal of the World Health Organization for ongoing and unpublished trials, and Clinicaltrials.gov/ for ongoing and other relevant trials on 15 February 2016 (Appendix 4). We also checked the reference lists of all the trials identified by the above methods.
We contacted the main author of NCT00323973 to obtain further details about the potentially published trial.
Data collection and analysis
We conducted data collection and analysis of data according to methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two authors (AMC, JK) independently assessed each reference to see whether it met the inclusion criteria. Any disagreements were resolved through consensus.
Data extraction and management
Two authors (AMC, JK) independently extracted data from the selected trials using a standardised data extraction form. Any disagreements were resolved through consensus. For the first version of this Cochrane review, Dr Viana Zuza Diniz was contacted, and sent us the full text of her PhD thesis (Diniz 2004).
Assessment of risk of bias in included studies
All review authors independently assessed the risk of bias of the trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following domains, using the following definitions.
Generation of the allocation sequence
Low risk if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice was considered adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear risk if the trial was described as randomised, but the method used for the generation of allocation sequence was not described.
High risk if a system involving dates, names, or admittance numbers was used for the allocation of participants.
Allocation concealment
Low risk if the allocation of participants involved a central independent unit, on‐site locked computer, identical looking, numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear risk if the trial was described as randomised, but the method used to conceal the allocation was not described.
High risk if the allocation sequence was known to the investigators who assigned participants, or if the study was quasi‐randomised.
Blinding (or masking)
We assessed each trial (as low, unclear, or high risk) with regard to the following types of blinding:
blinding of clinician (person delivering treatment) to treatment allocation;
blinding of participant to treatment allocation;
blinding of outcome assessor to treatment allocation.
Incomplete outcome data
Low risk if the numbers and reasons for dropouts and withdrawals in all intervention groups were described, or it was specified that there were no dropouts or withdrawals.
Unclear risk if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
High risk if the number or reasons for dropouts and withdrawals were not described.
We further examined the overall percentages of dropouts in each trial, per randomised arm, and evaluated whether intention‐to‐treat analysis was performed, or could be performed from the published information. We measured outcomes against all participants randomised. We did not impute values.
Selective outcome reporting
Low risk if pre‐defined or clinically relevant and reasonably expected outcomes were reported.
Unclear risk if not all pre‐defined or clinically relevant and reasonably expected outcomes were reported, or were not fully reported, or it was unclear whether data on these outcomes were recorded or not.
High risk if one or more clinically relevant and reasonably expected outcomes were not reported; data on these outcomes would be expected to have been recorded.
Other bias:
Low risk if the trial appears to be free of other components that could put it at risk of bias.
Unclear risk if the trial may or may not be free of other components that could put it at risk of bias.
High risk if there are other factors in the trial that could put it at risk of bias, e.g., early stopping, industry involvement, or an extreme baseline imbalance.
We considered trials at low risk of bias to be those that adequately generated their allocation sequence; had adequate allocation concealment, blinding, and handling of incomplete outcome data; were free of selective outcome reporting; and were free of other bias.
We considered trials in which we assessed at least one of the domains as having a high risk of bias or unclear risk of bias, to be trials with high risk of bias.
Measures of treatment effect
We calculated the relative risk (RR) with 95% confidence intervals (CI) for binary outcomes of all‐cause mortality and safety.
Dealing with missing data
We assessed the percentages of overall dropouts for each included trial and each randomised arm and evaluated whether an intention‐to‐treat analysis had been performed or could be performed with the available published information. We contacted Dr Viana Zuza Diniz who sent us the full text of her PhD thesis (Diniz 2004).
We conducted an intention‐to‐treat analysis. We measured outcomes against all participants randomised. We did not impute values.
Assessment of heterogeneity
We quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). When heterogeneity was detected (I² > 50%), we attempted to identify the possible causes of heterogeneity (Higgins 2011).
Assessment of reporting biases
We did not assess publication bias with a funnel plot because we only included three trials. For future updates, we will attempt to assess whether the review is subject to publication bias by using a funnel plot, if at least 10 trials are included.
Data synthesis
We pooled the results from the trials using Review Manager software (RevMan 2014). We summarized findings using a fixed‐effect model, according to the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011).
Summary of findings
We used the principles of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to assess the quality of the body of evidence associated with up to seven outcomes (Balshem 2011; Brozek 2011; Guyatt 2008; Guyatt 2011h). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence takes into consideration within study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g).
This updated Cochrane review assessed the quality of the body of evidence associated with these seven specific outcomes: all‐cause mortality, cardiac mortality at 30 days, time‐to‐heart decompensation, disease‐free period (at 30 days, 60 days, and 90 days), quality of life, adverse events.
Subgroup analysis and investigation of heterogeneity
In subsequent updates of this review, when sufficient data are available, we plan to carry out the following subgroup analyses:
Intervention;
New York Heart Association stage;
Conduction system disturbances;
Atrial and ventricular arrhythmias;
Chronic versus acute heart failure;
Heart failure with preserved ejection fraction: ≤ 40% versus > 40%.
We will only perform subgroup analysis for primary outcomes.
Sources of heterogeneity in the assessment of the primary outcome measure will be explored by subgroup analyses and meta‐regression analyses. The meta‐regression analyses will assess route of administration (intramuscular versus intravenous) and participants' characteristics. We will only conduct meta‐regression if at least 10 trials are included.
Sensitivity analysis
For future updates, we plan to conduct a sensitivity analysis comparing the results from all trials as follows:
Those trials with high methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') (Higgins 2011);
Those trials that performed intention‐to treat versus per‐protocol analyses.
We will also evaluate the risk of attrition bias, as estimated by the percentage of participants lost. Trials with a total attrition of more than 30%, or where differences between the groups exceed 10%, or both, will be excluded from meta‐analysis but will be included in the review.
Results
Description of studies
Results of the search
The initial search in 2011 identified 1125 unique records; we assessed six of these in full text. A seventh study was ongoing (NCT00323973). We excluded four studies (see Excluded studies).
The search in February 2016 identified 1041 new records, which resulted in 879 unique references after duplicates were removed, which we screened. After examining the titles and abstracts (857 references) and ongoing trials (22 registered studies), we excluded 878 references. We obtained full reprints of the remaining reference for a more detailed examination. Ultimately, we were able to find and include one new randomised clinical trial (Andrade 2012).
In total, this updated review includes three trials (four references) conducted in Brazil, and published between 2004 and 2012 (Andrade 2012; Botoni 2007; Diniz 2004). These trials involved 108 randomised participants. See Figure 2 for details.
2.
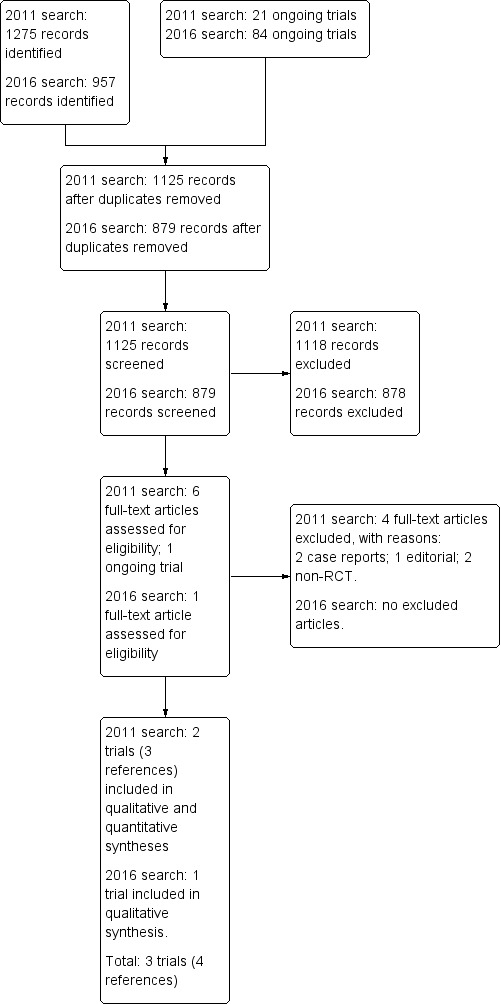
Study flow diagram first update 16 February 2016
Included studies
We provide a detailed description of the included trials in the Characteristics of included studies.
One trial reported no demographics, baseline, inclusion or exclusion criteria data (Andrade 2012). This trial involving 39 participants assessed rosuvastatin versus placebo. Information was obtained from a conference abstract. We contacted the trial author for details.
The following information came from Botoni 2007 and Diniz 2004. Overall, the mean age of the participants was 48.3 years (standard deviation (SD) 0.42). The percentage of male participants was 74.2% (SD 3.96). Botoni 2007 described New York Heart Association (NYHA) classes across both comparison groups. Fifty percent of the participants had NYHA class II/III. No participants had NYHA class IV (Botoni 2007). On the contrary, Diniz 2004 described NYHA class by comparison group. Fifteen participants in the carvedilol group had NYHA class between II to IV and the control group had NYHA class between II and III (Diniz 2004). The mean (SD) left ventricular ejection fraction (LVEF) for the carvedilol group in Botoni 2007 was 43.2 (19.9) versus 47.9 (15.3) for the control group. The mean (SD) LVEF for the carvedilol group was 0.26 (0.07) versus 0.24 (0.06) for control group in Diniz 2004.
Both trials assessed carvedilol by oral administration as the experimental intervention and placebo as the control group. Both were conducted using a parallel design with two arms, and both were conducted with out‐patients. The mean sample size was 34.5 (SD 6.36; range 30 to 39). One trial was reported in two publications (Diniz 2004). One trial reported that the sample size was calculated a priori (Botoni 2007), while the other did not (Diniz 2004). The included studies had follow–up periods ranging from four months to 29 weeks (Botoni 2007; Diniz 2004). One study was sponsored by drug company (Botoni 2007).
See Characteristics of included studies table for details.
Excluded studies
We excluded four references. Two were case reports (Bestetti 2011; Dávila 2002a), one reference was an editorial (Dávila 2008), and one was a non‐randomised controlled trial (Issa 2010). See the Characteristics of excluded studies table.
Ongoing studies
We identified one ongoing study (NCT00323973). We contacted the lead author who advised us on three separate occasions that the publication of this trial was held up in the editorial phase due to missing data. See Characteristics of ongoing studies for details.
Risk of bias in included studies
Overall, all trials had a high risk of bias. See Figure 3 and Figure 4 for risk of bias graph and summary.
3.
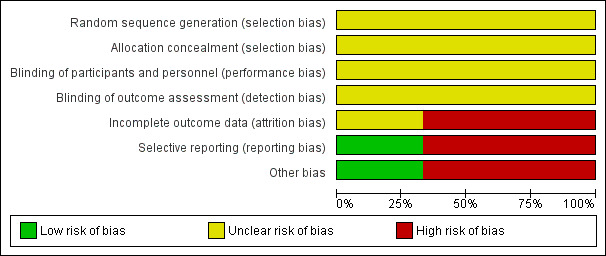
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
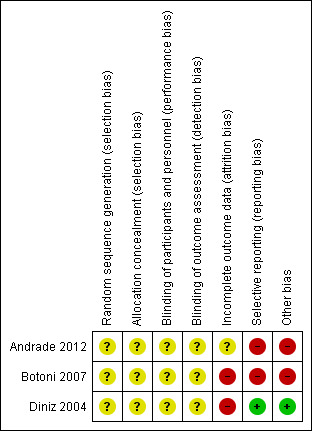
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.
Allocation
All trials were at unclear risk of bias for random sequence generation and allocation concealment domains.
Blinding
All trials were at unclear risk of bias for blinding of participants, personnel, and outcome assessors.
Incomplete outcome data
Andrade 2012 and was rated as having unclear risk of bias for this domain, while Diniz 2004 and Botoni 2007 were at a high risk of bias for this domain.
Selective reporting
One trial had a low risk of bias (Diniz 2004). Two trials were rated as having high risk of reporting bias (Andrade 2012; Botoni 2007).
Other potential sources of bias
One trial had a low risk of bias (Diniz 2004). Andrade 2012 and Botoni 2007 had bias in the presentation of data. Botoni 2007 received sponsorship from a drug company, therefore it was rated as having high risk of industry bias.
Effects of interventions
Summary of findings for the main comparison. Carvedilol compared with placebo for heart failure in patients with Chagas cardiomyopathy.
| Carvedilol compared with placebo for heart failure in patients with Chagas cardiomyopathy | ||||||
| Patient or population: heart failure in patients with Chagas cardiomyopathy Settings: outpatients Intervention: carvedilol Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Carvedilol | |||||
| All‐cause mortality Follow‐up: 4 to 6 months | 86 per 1000 | 59 per 1000 (10 to 333) | RR 0.69 (0.12 to 3.88) | 69 (2 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Cardiac mortality at 30 days | See comment | See comment | Not estimable | 69 (2 studies) | See comment | No trials reported this outcome |
| Time‐to‐heart decompensation | See comment | See comment | Not estimable | 69 (2 studies) | See comment | No trials reported this outcome |
| Disease‐free period (at 30, 60, and 90 days) | See comment | See comment | Not estimable | 69 (2 studies) | See comment | No trials reported this outcome. |
| Overall survival | See comment | See comment | Not estimable | 39 (1 study) | ⊕⊝⊝⊝ very low1,3 | Diniz 2004 only reported P = 0.525 |
| Quality of life assessed with the Minnesota Living With Heart Failure Questionnaire (MLHFQ). The total score for the 21 items ranges between 0 and 105. A lower MLHFQ score indicates less effect of heart failure on a patient’s quality of life. Follow‐up: 6 months | The mean quality of life in the intervention group was 14.74 lower (24.75 to 4.73 lower) | 30 (1 study) | ⊕⊝⊝⊝ very low1,3 | |||
| Adverse events Follow‐up: 6 months | 867 per 1000 | 797 per 1000 (581 to 1000) | RR 0.92 (0.67 to 1.27) | 30 (1 study) | ⊕⊝⊝⊝ very low1,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to limitations in design and execution: random sequence generation, allocation concealment and blinding at any level: unclear risk of bias. 2 Downgraded two levels due to imprecision. The sample size was very small (N = 69) and the number of events was very low (N = 5).
3 Downgraded two levels due to imprecision. The sample size was very small (N = 30) with wide confidence interval for the QoL outcome.
4 Downgraded two levels due to imprecision. The sample size was very small (N = 30) and the number of events was very low (N = 25).
Summary of findings 2. Rosuvastatin versus placebo for treating heart failure in patients with Chagas cardiomyopathy.
| Patient or population: chronic heart failure in Chagas cardiomyopathy patients Settings: unknown Intervention: rosuvastatin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Rosuvastatin | |||||
| All‐cause mortality | See comments | See comments | See comments |
39 (1 study) |
See comments | Trial did not assess this outcome |
| Cardiac mortality at 30 days | See comments | See comments | See comments |
39 (1 study) |
See comments | Trial did not assess this outcome |
| Time to heart decompensation | See comments | See comments | See comments |
39 (1 study) |
See comments | Trial did not assess this outcome |
| Disease‐free period at 30, 60 and 90 days | See comments | See comments | See comments |
39 (1 study) |
See comments | trial did not assess this outcome |
| Quality of life | See comments | See comments | See comments |
39 (1 study) |
⊕⊝⊝⊝ very low1,2 |
Trial did not state effect size |
| Adverse events | See comments | See comments | See comments |
39 (1 study) |
See comments | Trial did not assess this outcome |
| Hospital readmissions (heart failure‐ or adverse event‐related) | See comments | See comments | See comments |
39 (1 study) |
See comments | Trial did not assess this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to limitations in design and execution: random sequence generation, allocation concealment, and blinding at any level: unclear risk of bias. 2 Downgraded two levels due to imprecision. The sample size was very small (N = 39).
The results were based on three trials involving 108 participants (Andrade 2012; Botoni 2007; Diniz 2004). See Table 1 and Table 2.
Primary outcomes
The two trials (69 participants) that assessed carvedilol versus placebo reported only one of our primary outcomes of interest (Botoni 2007; Diniz 2004). No data were available on the other outcomes (30‐day cardiovascular mortality, time‐to‐heart decompensation, disease‐free period at 30, 60, and 90 days). Andrade 2012 assessed none of our primary outcomes.
All‐cause mortality
Carvedilol versus placebo
Meta‐analysis of two trials (69 participants) found a lower proportion of all‐cause mortality in the carvedilol group than the placebo group (2/34 (5.88%) versus 3/35 (8.57%); RR 0.69; 95% CI 0.12 to 3.88; I² = 0%; Analysis 1.1; very low‐quality evidence; Botoni 2007; Diniz 2004).
1.1. Analysis.
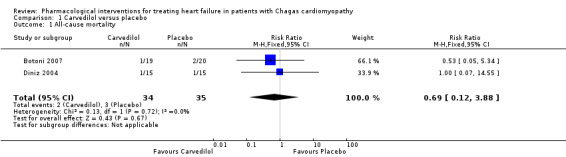
Comparison 1 Carvedilol versus placebo, Outcome 1 All‐cause mortality.
Secondary outcomes
Overall survival
Carvedilol versus placebo
One trial (30 participants) found similar overall survival results between the carvedilol and placebo groups (P = 0.525; very low‐quality evidence; Diniz 2004).
Hospital readmissions
One trial (30 participants) found similar hospital readmission results between the carvedilol and placebo groups (4/15 (26.66%) versus 4/15 (26.66%); RR 1.00; 95% CI 0.31 to 3.28; P = 1.0; Analysis 1.2; very low‐quality evidence; Diniz 2004).
1.2. Analysis.
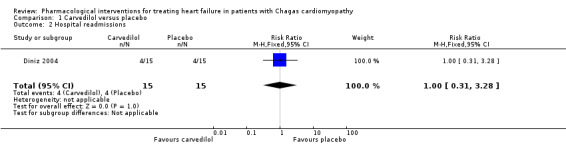
Comparison 1 Carvedilol versus placebo, Outcome 2 Hospital readmissions.
Quality of life
Carvedilol versus placebo
Due to inconsistency in measurement units between the two trials assessing this outcome, we were unable to combine the results from these trials.
Botoni 2007 (39 participants) assessed this outcome with the Medical Outcomes Study 36‐item short‐form health survey (SF‐36). This scale includes one multi‐item scale that assesses eight health concepts and 36 items (Ware 1992). Each item is quantified from 0 (worst) to 100 (better). Botoni 2007 found inconclusive differences between the carvedilol and placebo groups for functional capacity, physical limitation, pain, general state, vitality, social aspects, mental health, and emotional aspects.
Diniz 2004 assessed quality of life with the Minnesota Living With Heart Failure Questionnaire (MLHFQ). There are 21 items; each item is scored from 0 to 5, with a total range between 0 and 105. A lower MLHFQ score indicates less effect of heart failure on a patient’s quality of life (Pietri 2004). There is very low‐quality evidence that the effects on quality of life are inconclusive when carvedilol is compared with placebo (MD ‐14.74; 95% IC ‐24.75 to ‐4.73; 30 participants; Analysis 1.3).
1.3. Analysis.
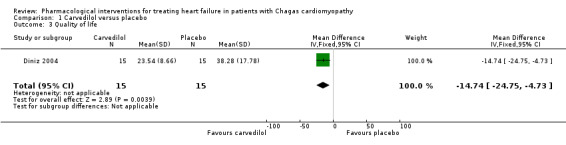
Comparison 1 Carvedilol versus placebo, Outcome 3 Quality of life.
Rosuvastatin versus placebo
Andrade 2012 (39 participants) reported no improvement in terms of quality of life. Trial authors provided no effect size.
Adverse events
Carvedilol versus placebo
One trial found a 'significant' reduction in systolic and diastolic blood pressure, and changes in renal function and serum electrolytes in both groups, but provided but no data. It also reported reductions in heart rate in both the carvedilol and placebo groups, but there was no recorded episode of symptomatic bradycardia (Botoni 2007). On the other hand, Diniz 2004 found uncertainty in the difference in adverse events between the carvedilol and placebo groups (12/15 (80%) and 13/15 (86.7%); RR 0.92; 95% CI 0.67 to 1.27; Analysis 1.4; very low‐quality evidence).
1.4. Analysis.
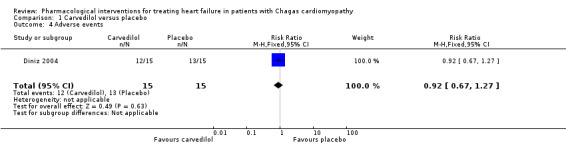
Comparison 1 Carvedilol versus placebo, Outcome 4 Adverse events.
Discussion
Summary of main results
This updated Cochrane review of pharmacological interventions for treating heart failure in patients with Chagas cardiomyopathy included a total of three trials (108 participants), all of which were conducted in Brazil. Two of the trials compared carvedilol with placebo (69 participants; Botoni 2007; Diniz 2004), and one compared rosuvastatin with placebo (39 participants; Andrade 2012). Our GRADE assessment found very low‐quality evidence on the effects of either carvedilol or rosuvastatin for treating heart failure in Chagas cardiomyopathy. All of the trials were small and had a high risk of bias. A drug company sponsored one trial (Botoni 2007).
We combined data from two trials, and found that carvedilol reduced all‐cause mortality over placebo, but the confidence intervals were wide and crossed the line of significance (Botoni 2007; Diniz 2004). It was not possible to pool data from Botoni 2007 and Diniz 2004 for overall survival or adverse events. Taking each trial separately, we found inconclusive results between the carvedilol and placebo groups regarding these outcomes. The trial comparing rosuvastatin and placebo reported no information. We found no conclusive results between carvedilol and placebo on hospital readmissions (Diniz 2004).
It was also not possible to pool quality of life (QoL) results; the effects across individual trials were inconclusive. One trial used the Minnesota Living With Heart Failure Questionnaire (MLHFQ) and reported that carvedilol significantly improved QoL more than placebo (Diniz 2004). On the contrary, Botoni 2007 found no conclusive results for QoL between the carvedilol with placebo groups when measured with the Medical Outcomes Study 36‐item short‐form health survey (SF‐36). Andrade 2012 did not report an effect size for QoL when rosuvastatin was compared with placebo.
None of the trials evaluated main clinical outcomes, such as cardiac mortality at 30 days, time‐to‐heart decompensation, disease‐free period (at 30, 60, and 90 days), adherence grade, or digoxin toxicity.
All evidence was graded as very low‐quality. See Table 1; Table 2 for details.
Overall completeness and applicability of evidence
This updated Cochrane review includes three small trials, which found inconclusive results between the effects of either carvedilol or rosuvastatin compared with placebo. All these issues yielded very low‐quality evidence (Ioannidis 2008a).
Only one meta‐analysis combined the data from two trials with very small sample sizes and a very low number of events. It has been shown that meta‐analyses that include a limited number of participants and events are prone to overestimate the estimate of effect (Ioannidis 2008a; Ioannidis 2008b; Thorlund 2011).
When dealing with such neutral results, we need to keep in mind that 'absence of evidence' is not 'evidence of absence' (Altman 1995; Fermi Paradox). The fact that this review did not detect conclusive results between the two intervention groups does not imply that placebo and carvedilol have the same mortality risk. The first possible explanation is failure to determine an appropriate sample size (Green 2002; Schulz 1995). In Freiman 1978, the authors suggested that "many of the therapies labelled as 'no different from control' in trials using inadequate samples, have not received a fair test" and that "concern for the probability of missing an important therapeutic improvement because of small sample sizes deserves more attention in the planning of clinical trials". In 1998, Moher, et al emphasized that "most trials with negative results did not have large enough sample sizes to detect a 25% or a 50% relative difference" (Moher 1998). Moreover, it has been suggested that the most important therapies adopted in clinical practice have shown more modest benefits (Kirby 2002).
Quality of the evidence
The GRADE approach was employed to interpret result findings and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager to create 'Summary of findings' tables (Table 1 and Table 2). The main source of bias in the included trials was the lack of detail in describing the generation of randomisation sequences and the concealment of allocation (Andrade 2012; Botoni 2007; Diniz 2004). Trials also lacked detail on their blinding processes. Our assessment of the risk of bias of the included studies has been previously described, and a summary can be found in Figure 3 and Figure 4. Included trials were generally considered to be at a high risk of bias. Uncertainty remains about possible harms from the interventions, due to a lack of detail in presenting safety data. All trials had very small sample sizes and a very low number of events, which reduced the precision and create wide confidence intervals. One trial is susceptible to high risk of industry bias (Botoni 2007).
Potential biases in the review process
In the process of performing a systematic review, there is a group of biases called significance‐chasing biases (Ioannidis 2010). This group includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials. This Cochrane review has a low risk of publication bias due to the thorough trial search process, through which we detected the primary source of Diniz 2004. Selective outcome reporting bias operates through suppression of information on specific outcomes and has similarities to study publication bias, in that 'negative' results remain unpublished (Ioannidis 2010). This Cochrane review found that included trials had a high risk of selective outcome reporting (Andrade 2012; Botoni 2007).
This update has two limitations. First, data from Andrade 2012 were gathered from a conference abstract. Second, we were not able to get information on the results of NCT00323973, which is completed, and compared bisoprolol versus placebo. However, we tried to reduce the negative impact of both limitations by contacting the main authors of Andrade 2012 and NCT00323973.
Authors' conclusions
Implications for practice.
This first update of the Cochrane review found very low‐quality evidence for the effects of either carvedilol or rosuvastatin, both compared with placebo, for treating heart failure in people with Chagas disease. The results were based on three trials with a high risk of bias, involving 108 participants.
Therefore, current evidence neither supports nor refutes prescription of these medications for people suffering from heart failure associated with Chagas cardiomyopathy. Until randomised clinical trials provide evidence of a treatment effect, and the trade‐off between potential benefits and harms is established, policy‐makers, clinicians, and academics should be cautious when recommending or administering either carvedilol or rosuvastatin for the treatment of heart failure in people with Chagas disease.
This updated Cochrane review does not provide evidence for other conventional pharmacological interventions for treating heart failure in people with Chagas cardiomyopathy.
Implications for research.
This updated Cochrane review has highlighted a need for large, well‐designed, high‐quality randomised trials to assess the benefits and harms of pharmacological interventions for treating heart failure in people with Chagas cardiomyopathy. The potential trials should include main clinical outcomes (patient‐oriented outcomes) such as all‐cause mortality, quality of life, overall survival, cardiac mortality at 30 days, time‐to‐heart decompensation, disease‐free period (at 30 days, 60 days, and 90 days), hospital readmissions (heart failure‐ or adverse event‐related), adherence grade, adverse events, and digoxin toxicity.
The trials should be conducted by independent researchers and reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement to improve the quality of reporting of efficacy, and obtain better reports of harms in clinical research (Ioannidis 2004; Moher 2010; Turner 2012). Future trials should be planned in accordance with the recommendations of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT; Chan 2013a; Chan 2013b) and the Foundation of Patient‐Centered Outcomes Research (Basch 2012; Gabriel 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 23 March 2016 | New citation required but conclusions have not changed | One new trial identified for inclusion. |
| 15 February 2016 | New search has been performed | Searches have been re‐run and are up‐to‐date to February 2016. |
Acknowledgements
We thank the Center of Disease Control (Atlanta, USA) for permission to reproduce Figure 1.
For this update, we thank Verônica Abdala, Gerente de Servicios de Información y Producción de Fuentes de Información from BIREME/OPS/OMS, who sent us the full text of the PhD thesis Diniz 2004. We want to express our gratitude to Ricardo Hidalgo, Daniel Simancas and Susana Nicola for their contributions in the first version of this Cochrane review.
Appendices
Appendix 1. Adverse events commonly associated with drugs used for treatment of heart‐failure
| Angiotensin converting enzyme inhibitors (ACE inhibitors).(Adorisio 2006)(Hamad 2007) | Angiotensin II receptor antagonists(Adorisio 2006) | Aldosterone receptor antagonists (Hamad 2007) | Inotropes (Hamad 2007) | Digitalis (Adorisio 2006) | Diuretics (Hamad 2007)(Adorisio 2006) |
Vasodilators (Adorisio 2006) |
Beta‐adrenoceptor antagonists(Adorisio 2006) |
Calcium antagonists(Adorisio 2006) |
| 1. By bradykinin potentiation (Dry cough (5% of patients), and Angioedema (0.1–0.2% of patients)). 2. Related to angiotensin suppression (hypotension, increase in serum creatinine and potassium). 3. Others are hypotension & electrolyte imbalance. |
1. Hypotension. 2. Worsening renal function and hyperkalaemia. | 1. Hyperkalemia. 2. Gynecomastia by spironolactone. | 1. Arrhythmic events (atrial fibrillation, atrial flutter, ventricular tachycardia, ventricular fibrillation). 2. Severe hypotension. | 1. Cardiac arrhythmias (e.g., ectopic and reentrant cardiac rhythms and heart block). 2. Gastrointestinal symptoms (e.g., anorexia, nausea, and vomiting). 3. Neurologic complaints (e.g., visual disturbances, disorientation, and confusion). |
1. Metabolic abnormalities:
1.1. Contraction alkalosis.
1.2. Hyponatremia.
1.3. Hypokalemia.
1.4. Increased blood urea nitrogen and creatinine.
1.5. Hypomagnesemia. 2. Hemodynamic: 2.1. Hypotension and/or diminished renal perfusion. |
1. Headache. 2. Dizziness. |
1. Fatigue and weakness.
2. Symptomatic bradycardia.
3. Hypotension. 4. "Administration of β‐blockers is contraindicated in patients with severe bronchospasm, symptomatic bradycardia, or advanced heart block in the absence of a pacemaker". 5. Bronchospam in patients with chronic obstructive pulmonary disease. |
1. Negative inotropic effect and reflex neurohormonal activation. 2. Peripheral and pulmonary oedema. |
Appendix 2. Medical glossary
| TERM | DEFINITION | SOURCE |
| Chagas Disease | Infection with the protozoan parasite Trypanosoma cruzi, a form of trypanosomiasis endemic in Central and South America. It is named after the Brazilian physician Carlos Chagas, who discovered the parasite. Infection by the parasite (positive serologic result only) is distinguished from the clinical manifestations that develop years later, such as destruction of parasympathetic ganglia; Chagas cardiomyopathy; and dysfunction of the oesophagus or colon. | MeSH Database PubMed |
| Carvedilol | Antioxidant with alpha as well as beta blocking activity; structure in first source | MeSH Database PubMed |
| Chagas cardiomyopathy | A disease of the cardiac muscle developed subsequent to the initial protozoan infection by Trypanosoma cruzi. Fewer than 10% of those infected develop acute illness such as myocarditis (mostly in children). The disease then enters a latent phase without clinical symptoms until about 20 years later. Myocardial symptoms of advanced Chagas disease include conduction defects (heart block) and cardiomegaly | MeSH Database PubMed |
| Digoxin | A cardiotonic glycoside obtained mainly from Digitalis lanata; it consists of three sugars and the aglycone digoxigenin. Digoxin has positive inotropic and negative chronotropic activity. It is used to control ventricular rate in atrial fibrillation and in the management of congestive heart failure with atrial fibrillation. Its use in congestive heart failure and sinus rhythm is less certain. The margin between toxic and therapeutic doses is small. | MeSH Database PubMed |
| Dilated cardiomyopathy | A form of cardiac muscle disease that is characterized by ventricular dilation, ventricular dysfunction, and heart failure. Risk factors include smoking, alcohol consumption, hypertension, infection, pregnancy, and mutations in the LMNA gene encoding Lamin type A, a nuclear lamina protein. | MeSH Database PubMed |
| Heart failure | A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. | MeSH Database PubMed |
| Left ventricular ejection fraction | Ejection fraction is a measurement of the percentage of blood leaving your heart each time it contracts. | http://www.mayoclinic.com/health/ejection‐fraction/AN00360 (accessed on 21 November 2011) |
| Renin‐Angiotensin‐System | A blood pressure regulating system of interacting components that include renin, angiotensinogen, angiotensin converting enzyme, angiotensin I, angiotensin II, and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha‐2 globulin produced by the liver, forming angiotensin I. Angiotensin‐converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidneys and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin‐converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the Kallikrein‐Kinin system. | MeSH Database PubMed |
| Trypanosoma Cruzi | The agent of South American trypanosomiasis or Chagas disease. Its vertebrate hosts are man and various domestic and wild animals. Insects of several species are vectors. | MeSH Database PubMed |
Appendix 3. Search strategies 2011
CENTRAL
#1 MeSH descriptor Chagas Disease explode all trees #2 chagas* #3 trypanosom* #4 MeSH descriptor Trypanosomiasis, this term only #5 cruzi #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Heart Failure explode all trees #8 heart next failure* #9 cardiac next failure* #10 myocardial next failure* #11 heart next incompet* #12 cardi* next incompet* #13 myocard* next incompet* #14 heart next insufficien* #15 cardi* next insufficien* #16 myocard* next insufficien* #17 cardi* next shock #18 myocard* next shock #19 heart next arrest* #20 cardi* next arrest* #21 myocard* next arrest* #22 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #23 (#16 OR #17 OR #18 OR #19 OR #20 OR #21) #24 (#22 OR #23) #25 (#6 AND #24)
MEDLINE
1. exp Chagas Disease/ 2. chagas*.tw. 3. cruzi*.tw. 4. trypanosom*.tw. 5. Trypanosomiasis/ 6. or/1‐5 7. exp Heart Failure/ 8. ((cardi* or heart* or myocard*) adj2 (failure* or incompet* or insufficien* or shock or arrest*)).tw. 9. 7 or 8 10. 6 and 9 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. drug therapy.fs. 16. randomly.ab. 17. trial.ab. 18. groups.ab. 19. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp animals/ not humans.sh. 21. 19 not 20 22. 10 and 21
EMBASE
1. Chagas disease/ 2. cardiomyopathy/ 3. chagas*.tw. 4. cruzi*.tw. 5. trypanosomiasis/ 6. trypanosom*.tw. 7. or/1‐6 8. exp heart failure/ 9. ((cardi* or heart* or myocard*) adj2 (failure* or incompet* or insufficien* or shock or arrest*)).tw. 10. 8 or 9 11. 7 and 10 12. random$.tw. 13. factorial$.tw. 14. crossover$.tw. 15. cross over$.tw. 16. cross‐over$.tw. 17. placebo$.tw. 18. (doubl$ adj blind$).tw. 19. (singl$ adj blind$).tw. 20. assign$.tw. 21. allocat$.tw. 22. volunteer$.tw. 23. crossover procedure/ 24. double blind procedure/ 25. randomized controlled trial/ 26. single blind procedure/ 27. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. (animal/ or nonhuman/) not human/ 29. 27 not 28 30. 11 and 29 31. limit 30 to embase
Web of Science
1. TS=chagas* 2. TS=cruzi* 3. TS=trypanosom* 4. 3 OR 2 OR 1 5. TS=((cardi* or heart* or myocard*) SAME (failure* or incompet* or insufficien* or shock or arrest*)) 6. 4 AND 5
LILACS
(heart or cardiac) and failure [Words] or "HEART FAILURE" [Subject descriptor] and chagas$ or cruzi$ or trypanosom$ [Words]
WHO ICTRP
chagas* and heart or chagas* and cardi*
clinicaltrials.gov
chagas and (heart or cardiac)
Appendix 4. Search strategies 2016
CENTRAL
#1 MeSH descriptor Chagas Disease explode all trees
#2 chagas*
#3 trypanosom*
#4 MeSH descriptor Trypanosomiasis, this term only
#5 cruzi
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH descriptor Heart Failure explode all trees
#8 heart next failure*
#9 cardiac next failure*
#10 myocardial next failure*
#11 heart next incompet*
#12 cardi* next incompet*
#13 myocard* next incompet*
#14 heart next insufficien*
#15 cardi* next insufficien*
#16 myocard* next insufficien*
#17 cardi* next shock
#18 myocard* next shock
#19 heart next arrest*
#20 cardi* next arrest*
#21 myocard* next arrest*
#22 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#23 (#16 OR #17 OR #18 OR #19 OR #20 OR #21)
#24 (#22 OR #23)
#25 (#6 AND #24)
MEDLINE OVID
The Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format has been applied (Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org)
1. exp Chagas Disease/
2. chagas*.tw.
3. cruzi*.tw.
4. trypanosom*.tw.
5. Trypanosomiasis/
6. or/1‐5
7. exp Heart Failure/
8. ((cardi* or heart* or myocard*) adj2 (failure* or incompet* or insufficien* or shock or arrest*)).tw.
9. 7 or 8
10. 6 and 9
11. randomized controlled trial.pt.
12. controlled clinical trial.pt.
13. randomized.ab.
14. placebo.ab.
15. drug therapy.fs.
16. randomly.ab.
17. trial.ab.
18. groups.ab.
19. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. exp animals/ not humans.sh.
21. 19 not 20
22. 10 and 21
EMBASE OVID
The Cochrane RCT filter for OVID EMBASE has been applied (Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org)
1. Chagas disease/
2. cardiomyopathy/
3. chagas*.tw.
4. cruzi*.tw.
5. trypanosomiasis/
6. trypanosom*.tw.
7. or/1‐6
8. exp heart failure/
9. ((cardi* or heart* or myocard*) adj2 (failure* or incompet* or insufficien* or shock or arrest*)).tw.
10. 8 or 9
11. 7 and 10
12. random$.tw.
13. factorial$.tw.
14. crossover$.tw.
15. cross over$.tw.
16. cross‐over$.tw.
17. placebo$.tw.
18. (doubl$ adj blind$).tw.
19. (singl$ adj blind$).tw.
20. assign$.tw.
21. allocat$.tw.
22. volunteer$.tw.
23. crossover procedure/
24. double blind procedure/
25. randomized controlled trial/
26. single blind procedure/
27. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28. (animal/ or nonhuman/) not human/
29. 27 not 28
30. 11 and 29
31. limit 30 to embase
ISI Web of Science
No RCT filter has been applied to this search. The reasonably low number of hits does not justify the risk of missing relevant papers by applying a RCT filter which has not been reliably tested and verified in its sensitivity and precision.
# 6 #5 AND #4
# 5 TS=((cardi* or heart* or myocard*) SAME (failure* or incompet* or insufficien* or shock or arrest*))
# 4 #3 OR #2 OR #1
# 3 TS=trypanosom*
# 2 TS=cruzi*
# 1 TS=chagas*
LILACS
(heart or cardiac) and failure [Words] or "HEART FAILURE" [Subject descriptor] and chagas$ or cruzi$ or trypanosom$ [Words]
WHO ICTRP Search Portal
Search string: chagas* and heart or chagas* and cardi*
Clinicaltrials.gov
Search string: chagas and (heart or cardiac)
Data and analyses
Comparison 1. Carvedilol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.88] |
| 2 Hospital readmissions | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.28] |
| 3 Quality of life | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐14.74 [‐24.75, ‐4.73] |
| 4 Adverse events | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrade 2012.
| Methods |
|
|
| Participants | Randomised: 39
Trial authors did not report information on: age, gender, inclusion or exclusion criteria, or New York Heart Association stage. |
|
| Interventions |
Cointervention: not given. |
|
| Outcomes |
Trial authors did not classify their outcomes as primary or secondary. |
|
| Notes |
We sent an e‐mail to the trial author on 23 January 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "... we randomized..." (Page 90). Insufficient information to permit judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "...double‐blind, placebo‐controlled..." (page 90) Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "...double‐blind, placebo‐controlled..." (page 90) Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | High risk | The study report failed to include results for a key outcome that would be expected to have been reported for such a study |
| Other bias | High risk | Bias in the presentation of the data. |
Botoni 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was achieved by each patient selecting an envelope that contained a number. The number was sent to the pharmacist, who provided the appropriate medication box to each patient. The medication container was identified only by each patient's name." (page 544e2) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was achieved by each patient selecting an envelope that contained a number. The number was sent to the pharmacist, who provided the appropriate medication box to each patient. The medication container was identified only by each patient's name." (page 544e2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “…the patients were assigned in a double blind fashion to receive either placebo or carvedilol... each patient selecting an envelope that contained a number. The number was sent to the pharmacist, who provided the appropriate medication box to each patient. The medication container was identified only by each patient's name." (page 544e2) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of participants before randomisation: 7.1% (3/42) Lost post‐randomisation: 24% (3/39) Carvedilol group: 5.2% (1/19) Placebo group: 10% (2/20) Imbalance between comparison groups: 4.8% Quote: “Three patients were lost in phase 1, each because of sudden death, poorly controlled ventricular tachycardia, and noncompliance. Of the 39 patients who entered phase II, 20 were randomised to receive placebo and 19 were randomised to receive carvedilol. Two patients from the placebo group were lost, each because of death caused by intractable HF and intolerable symptoms. One patient from the carvedilol group died suddenly." (page 544e5‐544e6) |
| Selective reporting (reporting bias) | High risk | The study report failed to include results for a key outcome that would be expected to have been reported for such a study. |
| Other bias | High risk | Industry bias |
Diniz 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss post‐randomisation: 16.6%. Comment: this trial had a small sample size; therefore, 16.6% could be considered a high loss of participants. |
| Selective reporting (reporting bias) | Low risk | This trial included four important outcomes: mortality, adverse events, quality of life, and safety. |
| Other bias | Low risk | ‐ |
SD = standard deviation
≥ = greater than or equal to
≤ = less than or equal to
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bestetti 2011 | Case report. |
| Dávila 2002a | Case report. |
| Dávila 2008 | Editorial. |
| Issa 2010 | Non‐randomised clinical trial. |
Characteristics of ongoing studies [ordered by study ID]
NCT00323973.
| Trial name or title |
|
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | July 2003. |
| Contact information | Carlos A Morillo, MD, FRCPC. Fundación Cardiovascular de Colombia. |
| Notes |
We contacted the lead author on 19 November 2014. |
Contributions of authors
AMC took the lead in writing up the Cochrane Review. JK: revised the Cochrane Review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Iberoamerican Cochrane Network, Spain.
Academic
-
Cochrane Heart Group, UK.
Academic.
Declarations of interest
In 2004 Arturo Martí‐Carvajal was employed by Eli Lilly to run a four‐hour workshop on 'how to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007 Arturo Martí‐Carvajal was employed by Merck to run a four‐hour workshop on 'how to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Joey Kwong has no known conflict of interest
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrade 2012 {published data only}
- Andrade MV, Boas FV, Daltro C, Carvalho C, Bernardes A, Nascimento T, et al. Effects of administration of short‐term rosuvastatin in patients with heart failure due to Chagas disease etiology: A randomized, placebo‐controlled trial. Cardiovascular Therapeutics 2012;30(Suppl 1):90. [Google Scholar]
Botoni 2007 {published data only}
- Botoni FA, Poole‐Wilson PA, Ribeiro AL, Okonko DO, Oliveira BM, Pinto AS, et al. A randomized trial of carvedilol after renin‐angiotensin system inhibition in chronic Chagas cardiomyopathy. American Heart Journal 2007;153(4):544.e1‐8. [PUBMED: 17383291] [DOI] [PubMed] [Google Scholar]
Diniz 2004 {published data only}
- Diniz RVZ. Effect and tolerability of the carvedilol in chronic symptomatic Chagasic cardiomyopathy [Efeito e tolerabilidade do carvedilol no tratamento de miocardiopatia chagásica crônica sintomática: estudo duplo‐cego, controlado com placebo [PhD Thesis]]. São Paulo, Brazil, Universidade Federal de São Paulo 2004. [419430 ]
- Diniz RVZ, Viegas RFM, Amori JML, Pulz C, Carvalho ACDC, Almeida DR. [Impacto clínico da utilização do beta‐bloqueador carvedilol na miocardiopatia chagásica crônica sintomática: estudo duplo‐cego, randomizado, controlado com placebo]. Arquivos Brasileiros de Cardiologia. 2005; Vol. 84:66. [DOI: 10.1590/S0066-782X2005000100014] [DOI]
References to studies excluded from this review
Bestetti 2011 {published data only}
- Bestetti RB, Otaviano AP, Cardinalli‐Neto A, Rocha BF, Theodoropoulos TA, Cordeiro JA. Effects of B‐Blockers on outcome of patients with Chagas' cardiomyopathy with chronic heart failure. International Journal of Cardiology 2011;151:205‐8. [PUBMED: 20591516] [DOI] [PubMed] [Google Scholar]
Dávila 2002a {published data only}
- Dávila DF, Angel F, Arata de Bellabarba G, Donis JH. Effects of metoprolol in chagasic patients with severe congestive heart failure. International Journal of Cardiology 2002;85(2‐3):255‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dávila 2008 {published data only}
- Dávila DF, Donis JH, Torres A, Gottberg CF, Ramoni‐Perazzi P, Arata de Bellabarba G, et al. Beta‐adrenergic blockers in chronic systolic heart failure secondary to Chagas' disease. International Journal of Cardiology 2008;128(1):1‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Issa 2010 {published data only}
- Bocchi EA, Cruz F, Guimaraes G, Pinho Moreira LF, Issa VS, Ayub Ferreira SM, et al. Long‐term prospective, randomized, controlled study using repetitive education at six‐month intervals and monitoring for adherence in heart failure outpatients: the REMADHE trial. Circulation. Heart Failure 2008;1(2):115‐24. [PUBMED: 19808281] [DOI] [PubMed] [Google Scholar]
- Issa VS, Amaral AF, Cruz FD, Ferreira SM, Guimaraes GV, Chizzola PR, et al. Beta‐blocker therapy and mortality of patients with Chagas cardiomyopathy: a subanalysis of the REMADHE prospective trial. Circulation. Heart Failure 2010;3(1):82‐8. [PUBMED: 19933408] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00323973 {published data only}
- Morillo CA. A randomized double‐blind placebo force‐titration controlled study with bisoprolol in patients with chronic heart failure secondary to Chagas´ cardiomyopathy. https://clinicaltrials.gov/ct2/show/results/NCT00323973 (accessed 29 January 2016). [DOI] [PMC free article] [PubMed]
Additional references
Abuhab 2013
- Abuhab A, Trindade E, Aulicino GB, Fujii S, Bocchi EA, Bacal F. Chagas' cardiomyopathy: the economic burden of an expensive and neglected disease. International Journal of Cardiology 2013;168:2375‐80. [PUBMED: 23465560] [DOI] [PubMed] [Google Scholar]
Acquatella 2008
- Acquatella H. Predicting heart failure and mortality in chronic Chagas' heart disease. A novel disorder in Spain. Revista Española de Cardiología 2008;61(2):105‐7. [PUBMED: 18364176] [PubMed] [Google Scholar]
Adorisio 2006
- Adorisio R, Luca L, Rossi J, Gheorghiade M. Pharmacological treatment of chronic heart failure. Heart Failure Reviews 2006;11(2):109‐23. [PUBMED: 16937030] [DOI] [PubMed] [Google Scholar]
Altman 1995
- Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ 1995;311:485. [PUBMED: 7647644] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrade 2011
- Andrade JP, Marin‐Neto JA, Paola AA, Vilas‐Boas F, Oliveira GM, Bacal F, et al. I Latin American guidelines for the diagnosis and treatment of Chagas cardiomyopathy [I Diretriz Latino‐Americana para o Diagnostico e Tratamento da Cardiopatia Chagasica]. Arquivos Brasileiros de Cardiologia 2011;97(2 Suppl 3):1‐48. [PUBMED: 21952638] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Basch 2012
- Basch E, Aronson N, Berg A, Flum D, Gabriel S, Goodman SN, et al. Methodological standards and patient‐centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307(15):1636‐40. [PUBMED: 22511692] [DOI] [PubMed] [Google Scholar]
Bauman 2006
- Bauman JL, Didomenico RJ, Galanter WL. Mechanisms, manifestations, and management of digoxin toxicity in the modern era. American Journal of Cardiovascular Drugs 2006;6(2):77‐86. [PUBMED: 16555861] [DOI] [PubMed] [Google Scholar]
Bern 2009
- Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clinical Infectious Diseases 2009;49(5):e52‐4. [PUBMED: 19640226] [DOI] [PubMed] [Google Scholar]
Bestetti 2013
- Bestetti RB, Otaviano AP, Fantini JP, Cardinalli‐Neto A, Nakazone MA, Nogueira PR. Prognosis of patients with chronic systolic heart failure: Chagas disease versus systemic arterial hypertension. International Journal of Cardiology 2013;168(3):2990‐1. [PUBMED: 23642596] [DOI] [PubMed] [Google Scholar]
Betestti 2014
- Bestetti RB, Otaviano AP, Fantini JP, Cardinalli‐Neto A, Nakazone MA, Nogueira PR. Mode of death in chronic systolic heart failure: Chagas cardiomyopathy versus systemic arterial hypertension. International Journal of Cardiology 2014;174(3):818‐9. [PUBMED: 24794968] [DOI] [PubMed] [Google Scholar]
Bimbi 2014
- Bimbi BJ, Unger P, Vandenbossche JL, Silance PG, Laethem Y. Chagas disease: don't forget it in Latin American patients with heart block!. Acta Cardiologica 2014;69(2):206‐8. [PUBMED: 24783476] [DOI] [PubMed] [Google Scholar]
Biolo 2010
- Biolo A, Ribeiro AL, Clausell N. Chagas cardiomyopathy ‐ where do we stand after a hundred years?. Progress in Cardiovascular Diseases 2010;52(4):300‐16. [PUBMED: 20109600] [DOI] [PubMed] [Google Scholar]
Bonney 2008
- Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many?. Current Molecular Medicine 2008;8(6):510‐8. [PUBMED: 18781958 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brozek 2011
- Brozek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy 2011;66(5):588‐95. [PUBMED: 21241318] [DOI] [PubMed] [Google Scholar]
Buckner 2010
- Buckner FS, Navabi N. Advances in Chagas disease drug development: 2009‐2010. Current Opinion in Infectious Diseases 2010;Sep 30:[Epub ahead of print]. [PUBMED: 20885320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrasco 2012
- Carrasco HJ, Segovia M, Llewellyn MS, Morocoima A, Urdaneta‐Morales S, Martinez C, et al. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Neglected Tropical Diseases 2012;6(6):e1707. [PUBMED: 22745843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013a
- Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [PUBMED: 23303884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013b
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [PUBMED: 23295957] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2012
- Costa J, Peterson AT. Ecological niche modelling as a tool for understanding distributions and interactions of vectors, hosts, and etiologic agents of Chagas disease. Advances in Experimental Medicine and Biology 2012;710:59‐70. [PUBMED: 22127886] [DOI] [PubMed] [Google Scholar]
Coura 2010
- Coura JR, Borges‐Pereira J. Chagas disease: 100 years after its discovery. A systemic review. Acta Tropica 2010;115(1‐2):5‐13. [PUBMED: 20382097] [DOI] [PubMed] [Google Scholar]
Cruz‐Pacheco 2012
- Cruz‐Pacheco G, Esteva L, Vargas C. Control measures for Chagas disease. Mathematical Biosciences 2012;237(1‐2):49‐60. [PUBMED: 22450034] [DOI] [PubMed] [Google Scholar]
Cunha‐Neto 2014
- Cunha‐Neto E, Chevillard C. Chagas disease cardiomyopathy: Immunopathology and genetics. Mediators of Inflammation 2014;Epub Aug 19:11. [PUBMED: 25210230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diniz 2010
- Diniz Lde F, Caldas IS, Guedes PM, Crepalde G, Lana M, Carneiro CM, et al. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrobial Agents and Chemotherapy 2010;54(7):2979‐86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobarro 2008
- Dobarro D, Gomez‐Rubin C, Sanchez‐Recalde A, Olias F, Bret‐Zurita M, Cuesta‐Lopez E, et al. Chagas' heart disease in Europe: an emergent disease?. Journal of Cardiovascular Medicine 2008;9(12):1263‐7. [PUBMED: 19001935] [DOI] [PubMed] [Google Scholar]
Dutra 2008
- Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Current Opinion in Infectious Diseases 2008;21(3):287‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dávila 2002b
- Dávila DF, Rossell O, Bellabarba GA. Pathogenesis of chronic Chagas heart disease: parasite persistence and autoimmune responses versus cardiac remodelling and neurohormonal activation. International Journal for Parasitology 2002;32(1):107‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dávila 2004
- Dávila DF, Donis JH, Torres A, Ferrer JA. A modified and unifying neurogenic hypothesis can explain the natural history of chronic Chagas heart disease. International Journal of Cardiology 2004;96(2):191‐5. [PUBMED: 15262032] [DOI] [PubMed] [Google Scholar]
Dávila 2005
- Dávila DF, Santiago JJ, Odreman WA. Vagal dysfunction and the pathogenesis of chronic Chagas disease. International Journal of Cardiology 2005;100(2):337‐9. [PUBMED: 15823646] [DOI] [PubMed] [Google Scholar]
Elias 2003
- Elias FE, Vigliano CA, Laguens RP, Levin MJ, Berek C. Analysis of the presence of Trypanosoma cruzi in the heart tissue of three patients with chronic Chagas' heart disease. The American Journal of Tropical Medicine and Hygiene 2003;68(2):242‐7. [PUBMED: 12641419] [PubMed] [Google Scholar]
Esper 2015
- Esper L, Talvani A, Pimentel P, Teixeira MM, Machado FS. Molecular mechanisms of myocarditis caused by Trypanosoma cruzi. Current Opinion in Infectious Diseases 2015;28(3):246‐52. [PUBMED: 25887609] [DOI] [PubMed] [Google Scholar]
Fermi Paradox
- Fermi Paradox. crystalinks.com/fermiparadox.html (accessed 22 January 2015).
Freiman 1978
- Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. New England Journal of Medicine 1978;299:690‐4. [PUBMED: 355881] [DOI] [PubMed] [Google Scholar]
Gabriel 2012
- Gabriel SE, Normand SL. Getting the methods right ‐ the Foundation of Patient‐Centered Outcomes Research. New England Journal of Medicine 2012;367(9):787‐90. [PUBMED: 22830434] [DOI] [PubMed] [Google Scholar]
Gascón 2007
- Gascón J, Albajar P, Cañas E, Flores M, Gómez i Prat J, Herrera RN, et al. Diagnosis, management and treatment of chronic Chagas' heart disease in areas where Trypanosoma cruzi infection is not endemic [Diagnóstico, manejo y tratamiento de la cardiopatíachagásica crónica en áreas donde la infección porTrypanosoma cruzi no es endémica]. Revista Española de Cardiología 2007;60(3):285‐93. [PUBMED: 17394874] [PubMed] [Google Scholar]
Gascón 2010
- Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non‐endemic countries. Acta Tropica 2010;115(1‐2):22‐7. [PUBMED: 19646412] [DOI] [PubMed] [Google Scholar]
Gironès 2005
- Gironès N, Cuervo H, Fresno M. Trypanosoma cruzi‐induced molecular mimicry and Chagas' disease. Current Topics in Microbiology and Immunology 2005;296:89‐123. [PUBMED: 16323421] [DOI] [PubMed] [Google Scholar]
Gironès 2007
- Gironès N, Carrasco‐Marin E, Cuervo H, Guerrero NA, Sanoja C, John S, et al. Role of Trypanosoma cruzi autoreactive T cells in the generation of cardiac pathology. Annals of the New York Academy of Sciences 2007;1107:434‐44. [PUBMED: 17804572] [DOI] [PubMed] [Google Scholar]
Granger 2009
- Granger BB, Ekman I, Granger CB, Ostergren J, Olofsson B, Michelson E, et al. Adherence to medication according to sex and age in the CHARM programme. European Journal of Heart Failure 2009;11(11):1092‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Green 2002
- Green SB. Design of randomized trials. Epidemiologic Reviews 2002;24:4‐11. [PUBMED: 12119855] [DOI] [PubMed] [Google Scholar]
Gurgel‐Goncalves 2012
- Gurgel‐Goncalves R, Galvao C, Costa J, Peterson AT. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modelling. Journal of Tropical Medicine 2012;2012:705326. [PUBMED: 22523500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Hamad 2007
- Hamad E, Mather PJ, Srinivasan S, Rubin S, Whellan DJ, Feldman AM. Pharmacologic therapy of chronic heart failure. American Journal of Cardiovascular Drugs 2007;7(4):235‐48. [PUBMED: 17696565] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higuchi 2003
- Higuchi ML, Benvenuti LA, Reis MM, Metzger M. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovascular Research 2003;60:96‐107. [PUBMED: 14522411 ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Ioannidis 2008a
- Ioannidis JP. Perfect study, poor evidence: interpretation of biases preceding study design. Seminars in Hematology 2008;45(3):160‐6. [PUBMED: 18582622] [DOI] [PubMed] [Google Scholar]
Ioannidis 2008b
- Ioannidis JP. Why most discovered true associations are inflated. Epidemiology 2008;9(5):640‐8. [PUBMED: 18633328] [DOI] [PubMed] [Google Scholar]
Ioannidis 2010
- Ioannidis JP. Meta‐research: the art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. [DOI] [PubMed] [Google Scholar]
Khatibzadeh 2013
- Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. International Journal of Cardiology 2013;168(2):1186‐94. [PUBMED: 23201083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirby 2002
- Kirby A, Gebski V, Keech AC. Determining the sample size in a clinical trial. The Medical Journal of Australia 2002;177:256‐7. [PUBMED: 12197821] [DOI] [PubMed] [Google Scholar]
Labarthe 1998
- Labathe DR. Chagas' disease and other cardiomyopathies. In: Labathe DR editor(s). Epidemiology and Prevention of Cardiovascular Diseases: A Global Challenge. 1st Edition. Maryland: Aspen, 1998:567‐84. [ISBN 0‐8342‐0659‐5] [Google Scholar]
Lee 2013
- Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. Lancet Infectious Diseases 2013;13:342‐8. [PUBMED: 23395248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Machado 2012
- Machado FS, Jelicks LA, Kirchhoff LV, Shirani J, Nagajyothi F, Mukherjee S, et al. Chagas heart disease report on recent developments. Cardiology in Review 2012;20(2):53‐65. [PUBMED: 22293860] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malik 2015a
- Malik LH, Singh GD, Amsterdam EA. The epidemiology, clinical manifestations, and management of Chagas heart disease. Clinical Cardiology 2015;38(9):565‐9. [PUBMED: 25993972] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malik 2015b
- Malik LH, Singh GD, Amsterdam EA. Chagas Heart Disease: An update. The American Journal of Medicine 2015;128(11):1251.e7‐9. [PUBMED: 26052027] [DOI] [PubMed] [Google Scholar]
Marin‐Neto 1998
- Marin‐Neto JA, Bromberg‐Marin G, Pazin‐Filho A, Simoes MV, Maciel BC. Cardiac autonomic impairment and early myocardial damage involving the right ventricle are independent phenomena in Chagas disease. International Journal of Cardiology 1998;65(3):261‐9. [PUBMED: 9740483] [DOI] [PubMed] [Google Scholar]
Marin‐Neto 2007
- Marin‐Neto JA, Cunha‐Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation 2007;115(9):1109‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Medei 2008
- Medei EH, Nascimento JH, Pedrosa RC, Carvalho AC. Role of autoantibodies in the physiopathology of Chagas' disease. Arquivos Brasileiros de Cardiologia 2008;91(4):257‐62, 281‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melton 2015
- Melton KD, Foil KJ, Yehle KS, Griggs RR. Heart failure in Hispanic Americans: Improving cultural awareness. The Journal for Nurse Practitioners 2015;11(2):207‐13. [DOI: ] [Google Scholar]
Mendez 2001
- Mendez GF, Cowie MR. The epidemiological features of heart failure in developing countries: a review of the literature. International Journal of Cardiology 2001;80(2):213‐9. [PUBMED: 11578717] [DOI] [PubMed] [Google Scholar]
Mills 2001
- Mills RM, Hobbs RE. Drug treatment of patients with decompensated heart failure. American Journal of Cardiovascular Drugs 2001;1(2):119‐25. [PUBMED: 14728041] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352:609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [PUBMED: 20332511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moncayo 2006
- Moncayo A, Ortiz Yanine MI. An update on Chagas disease (human American trypanosomiasis). Annals of Tropical Medicine and Parasitology 2006;100(8):663‐77. [PUBMED: 17227647] [DOI] [PubMed] [Google Scholar]
Moncayo 2009
- Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Memórias do Instituto Oswaldo Cruz 2009;104(Suppl 1):17‐30. [PUBMED: 19753454] [DOI] [PubMed] [Google Scholar]
Moncayo 2010
- Moncayo A. Carlos Chagas: biographical sketch. Acta Tropica 2010;115(1‐2):1‐4. [PUBMED: 19895782] [DOI] [PubMed] [Google Scholar]
Muñoz 2009
- Muñoz J, Coll O, Juncosa T, Vergés M, Pino M, Fumado V, et al. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clinical Infectious Diseases 2009;48(12):1736‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795‐801. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olivieri 2010
- Olivieri BP, Molina JT, Castro SL, Pereira MC, Calvet CM, Urbina JA, et al. A comparative study of posaconazole and benznidazole in the prevention of heart damage and promotion of trypanocidal immune response in a murine model of Chagas disease. International Journal of Antimicrobial Agents 2010;36(1):79‐83. [PUBMED: 20452188] [DOI] [PubMed] [Google Scholar]
OPS 2006
- Jannin J, Salvatella R. Quantitative estimation of Chagas Disease in the Americas [Estimación cuantitativa de la enfermedad de Chagas en Las Américas]. Organización Panamericana de la Salud. http://ops‐uruguay.bvsalud.org 2006 (accessed 5 July 2016).
Pietri 2004
- Pietri G, Ganse E, Ferrer M, Garin O, Wiklund I. MLHF. Minnesota living with heart failure questionnaire. User manual. 178.23.156.107:8085/Instruments_files/USERS/mlhf.pdf 2004 (accessed 27 January 2015).
Porta 2008
- Porta M. A Dictionary of Epidemiology. 5th Edition. New York: Oxford University Press, 2008. [Google Scholar]
Punukollu 2007
- Punukollu G, Gowda RM, Khan IA, Navarro VS, Vasavada BC. Clinical aspects of the Chagas' heart disease. International Journal of Cardiology 2007;115(3):279‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quiros 2006
- Quiros FR, Morillo CA, Casas JP, Cubillos LA, Silva FA. CHARITY: Chagas cardiomyopathy bisoprolol intervention study: a randomized double‐blind placebo force‐titration controlled study with Bisoprolol in patients with chronic heart failure secondary to Chagas cardiomyopathy [NCT00323973]. Trials 2006;7:21. [PUBMED: 16764726] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rassi 2000
- Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation 2007;115(9):1101‐8. [PUBMED: 17339568] [DOI] [PubMed] [Google Scholar]
Rassi 2006
- Rassi A Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. New England Journal of Medicine 2006;355(8):799‐808. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rassi Jr 2009
- Rassi Jr A, Rassi A, Marin‐Neto JA. Chagas heart disease: pathophysiologic mechanisms, prognostic factors and risk stratification. Memórias do Instituto Oswaldo Cruz 2009;104(Suppl 1):152‐8. [PUBMED: 19753470] [DOI] [PubMed] [Google Scholar]
Rassi Jr 2010
- Rassi A Jr, Rassi A, Marin‐Neto JA. Chagas disease. Lancet 2010;375(9723):1388‐402. [PUBMED: 20399979] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Reyes 2005
- Reyes PA, Vallejo M. Trypanocidal drugs for late stage, symptomatic Chagas disease (Trypanosoma cruzi infection). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004102.pub2] [DOI] [PubMed] [Google Scholar]
Ribeiro 2009
- Ribeiro CH, López NC, Ramírez GA, Valck CE, Molina MC, Aguilar L, et al. Trypanosoma cruzi calreticulin: a possible role in Chagas' disease autoimmunity. Molecular Immunology 2009;46(6):1092‐9. [PUBMED: 19108895] [DOI] [PubMed] [Google Scholar]
Ribeiro 2012a
Ribeiro 2012b
- Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO. Diagnosis and management of Chagas disease and cardiomyopathy. Nature Reviews Cardiology 2012;9(10):576‐89. [PUBMED: 22847166] [DOI] [PubMed] [Google Scholar]
Rossi 1991
- Rossi MA. The pattern of myocardial fibrosis in chronic Chagas' heart disease. International Journal of Cardiology 1991;30(3):335‐40. [PUBMED: 2055674] [DOI] [PubMed] [Google Scholar]
Schmunis 2010
- Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Tropica 2010;115(1‐2):14‐21. [PUBMED: 19932071] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Silva 2008
- Silva CP, Carlo CH, Oliveira Junior MT, Scipioni A, Strunz‐Cassaro C, Ramirez JA, et al. Why do patients with chagasic cardiomyopathy have worse outcomes than those with non‐chagasic cardiomyopathy?. Arquivos Brasileiros de Cardiologia 2008;91(6):358‐62. [PUBMED: 19142362] [DOI] [PubMed] [Google Scholar]
Soriano 2009
- Soriano Arandes A, Muñoz Gutierrez J, Vergés Navarro M, Castells Doménech C, Portús Vinyeta M, Gascón Brustenga J. Prevalence of Chagas disease in the Latin American immigrant population in a primary health centre in Barcelona (Spain). Acta Tropica 2009;112(2):228‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sousa 2015
- Sousa GR, Costa HS, Souza AC, Nunes MCP, Lima MMO, Rocha MOD. Health‐related quality of life in patients with Chagas disease: a review of the evidence. Revista Da Sociedade Brasileira De Medicina Tropical 2015;48:121‐8. [25992924] [DOI] [PubMed] [Google Scholar]
Strasen 2014
- Strasen J, Williams T, Ertl G, Zoller T, Stich A, Ritter O. Epidemiology of Chagas disease in Europe: many calculations, little knowledge. Clinical Research in Cardiology 2014;103(1):1‐10. [DOI] [PubMed] [Google Scholar]
Tanowitz 2009
- Tanowitz HB, Machado FS, Jelicks LA, Shirani J, Carvalho AC, Spray DC, et al. Perspectives on Trypanosoma cruzi‐induced heart disease (Chagas disease). Progress in Cardiovascular Diseases 2009;51(6):524‐39. [PUBMED: 19410685] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teixeira 2011
- Teixeira AR, Hecht MM, Guimaro MC, Sousa AO, Nitz N. Pathogenesis of Chagas' disease: Parasite persistence and autoimmunity. Clinical Microbiology Reviews 2011;24(3):592‐630. [PUBMED: 21734249] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PloS one 2011;6(10):e25491. [PUBMED: 22028777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Traina 2015
- Traina MI, Sanchez DR, Hernandez S, Bradfield JS, Labedi MR, Ngab TA, et al. Prevalence and impact of Chagas disease among Latin American immigrants with nonischemic cardiomyopathy in Los Angeles, California. Circulation‐Heart Failure 2015;8(5):938‐43. [PUBMED: 26206855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2012
- Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.MR000030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Urbina 2010
- Urbina J. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Tropica 2010;115(1‐2):55‐68. [PUBMED: 19900395] [DOI] [PubMed] [Google Scholar]
Veloso 2014
- Veloso HH. Incidence of sudden cardiac death in congestive heart failure: Chagas disease versus systemic arterial hypertension. International Journal of Cardiology 2014;175(1):175‐6. [PUBMED: 24852839] [DOI] [PubMed] [Google Scholar]
Villar 2002
- Villar JC, Villar LA, Marin‐Neto JA, Ebrahim S, Yusuf S. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003463] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
Yamagata 2006
- Yamagata Y, Nakagawa J. Control of Chagas disease. Advances in Parasitology 2006;61:129‐65. [PUBMED: 16735164] [DOI] [PubMed] [Google Scholar]
Zhang 1999
- Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. The Journal of Infectious Diseases 1999;180(2):480‐6. [PUBMED: 10395865 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hidalgo 2012
- Hidalgo R, Martí‐Carvajal AJ, Kwong JSW, Simancas‐Racines D, Nicola S. Pharmacological interventions for treating heart failure in patients with Chagas cardiomyopathy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD009077.pub2] [DOI] [PubMed] [Google Scholar]


