Fig. 5. Model of membrane constriction induced by remodeling of CHMP2A-CHMP3 filaments.
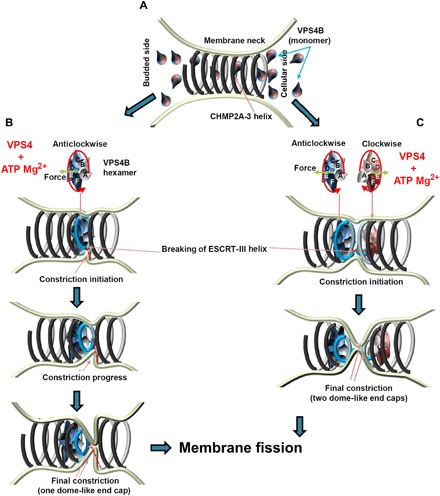
(A) CHMP2A-CHMP3 helical filaments assemble within a membrane neck structure such as a vesicle or virus bud or at the midbody. Currently, we do not know how many turns assemble in vivo. (B) VPS4 forms an asymmetric hexamer structure in the presence of ATP and Mg2+. This structure needs to assemble on functional ESCRT-III filaments, and ATP-driven rotation threads the substrate via its central pore. Because CHMP2A-CHMP3 polymer assembly is directional, the assembly of VPS4 acting clockwise or anticlockwise is likely to be important. The assembly of one VPS4 complex might be sufficient to induce CHMP2A-CHMP3 constriction, which often starts asymmetrically (middle). This can lead to membrane constriction and cleavage of the CHMP2A-CHMP3 filament. (C) Alternatively, two adjacent VPS4B complexes remodel CHMP2A-CHMP3 filaments acting clockwise and anticlockwise, thereby leading to the generation of two dome-like end caps and cleavage of the CHMP2A-CHMP3 filament. In both scenarios, constriction of CHMP2A-CHMP3 could prime the site for fission, and cleavage of the filament might play an important role in tension release as proposed (53).
