Fig. 4. GABAA receptor trafficking was disrupted by alterations of GABARAPL2 due to autophagy deficiency.
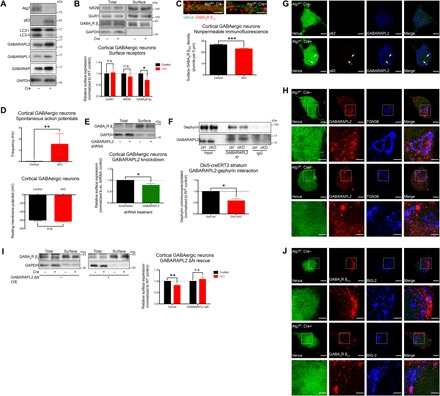
(A) Atg7 deletion in cultured cortical GABAergic interneurons (DIV15) derived from the MGEs of Atg7flox/flox embryos followed by infection with lentivirus expressing Venus reporter with or without Cre recombinase. Disrupted LC3 processing and accumulations of p62 and GABARAPs were observed as a result of autophagy deficiency induced by Atg7 deletion. (B) A significant reduction of surface GABAA receptors was observed in Atg7 cKO cultured cortical GABAergic interneurons, whereas NMDA and AMPA receptors were unchanged in both total and surface fractions. n = 4 independent experiments using distinct preparations of primary cultured neurons. (C) Immunofluorescence under nonpermeable conditions showed a significant reduction of surface GABAA receptors in Atg7 cKO cultured cortical GABAergic interneurons. Venus expression (green fluorescence) was used as an indicator of infected neurons. n = 30 cells analyzed per genotype. (D) A significant increase in the frequency of spontaneous action potentials fired by Atg7 cKO cultured cortical GABAergic interneurons was observed compared to controls (top). n > 25 cells per genotype; data shown for n = 10 and 9 cells for control and Atg7 cKO, respectively, from three distinct preparations of primary cultured neurons, which fired spontaneous action potentials. Resting membrane potential was not altered by Atg7 deletion in cultured cortical GABAergic interneurons (bottom). n > 30 cells per genotype from three distinct preparations of primary cultured neurons. (E) Gabarapl2 knockdown in WT cultured cortical GABAergic interneurons resulted in a significant reduction of surface GABAA receptors. n = 3 independent experiments using distinct preparations of primary cultured neurons. (F) GABARAPL2-gephyrin interaction was significantly reduced in the brains of Dlx5-creERT2 (striatum) Atg7 cKO mice (see fig. S4D). Quantifications shown represent the amount of gephyrin coimmunoprecipitated normalized by total gephyrin and amount of GABARAPL2 immunoprecipitated from individual samples. n = 3 independent experiments using tissue homogenates from distinct animals. (G) GABARAPL2 was mislocalized to p62+ aggregates formed in Atg7 cKO cultured cortical GABAergic interneurons in a similar manner as in affected neurons of Atg7 cKO brains. (H) GABARAPL2 was observed by immunofluorescence to localize to the TGN marked by TGN38 in controls, but this localization was reduced in Atg7 cKO cultured cortical GABAergic interneurons as the protein became mislocalized into foci. Venus signal was reduced in merge images to allow better visualization of signals from other channels. (I) GABARAPL2 ΔN overexpression (O/E) reversed the reduction of surface GABAA receptors observed in Atg7 cKO cultured cortical GABAergic interneurons. n = 5 independent experiments using distinct preparations of primary cultured neurons. (J) Intracellular accumulations of GABAA receptors were observed in BIG-2+ structures of Atg7 cKO cultured cortical GABAergic interneurons but not in controls. Data are presented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, paired (B, E, F, and I) or unpaired (C) two-tailed Student’s t test or Kolmogorov-Smirnov test (D). Scale bars, 2 μm (C), 5 μm (G), 5 μm (H and J), and 2 μm (insets).
