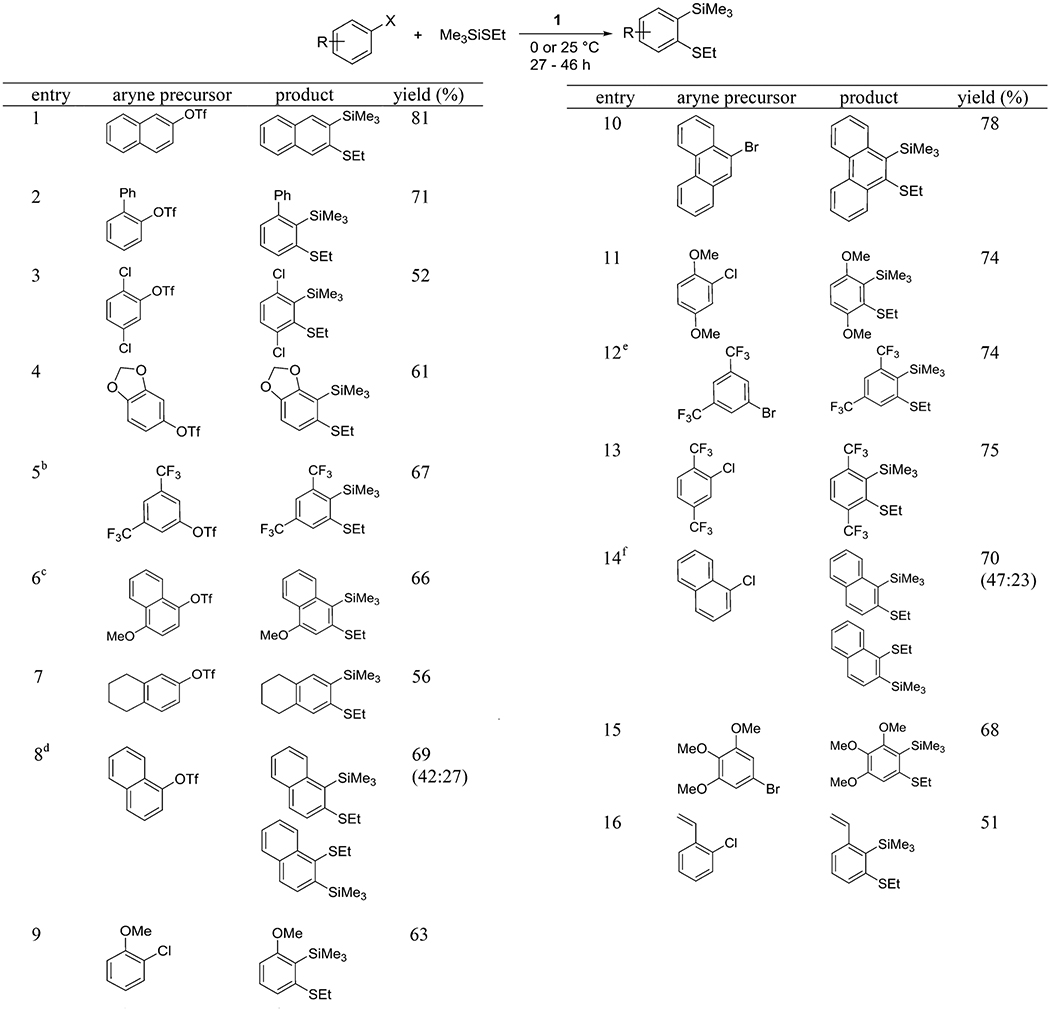
Table 4.
Insertion of Arynes into Si–S Bondsa
ArX (1 equiv), Me3SiSEt (3 equiv), base (2 equiv), diethyl ether/C6H12 (1:1, 3 mL) for ArOTf, diethyl ether (3 mL) for ArHal, 0.5 mmol scale, yields are isolated yields. See the Supporting Information for details.
Product contains 4% of an isomeric impurity.
Crude mixture contains another isomer (4.2:1), which was separated during chromatography.
Yields are of pure isomers, separated by column chromatography.
Reaction mixture contained another isomer (16:1), which was separated by column chromatography.
Yields are of pure isomers, separated by column chromatography. Crude isomer ratio 1.9:1.