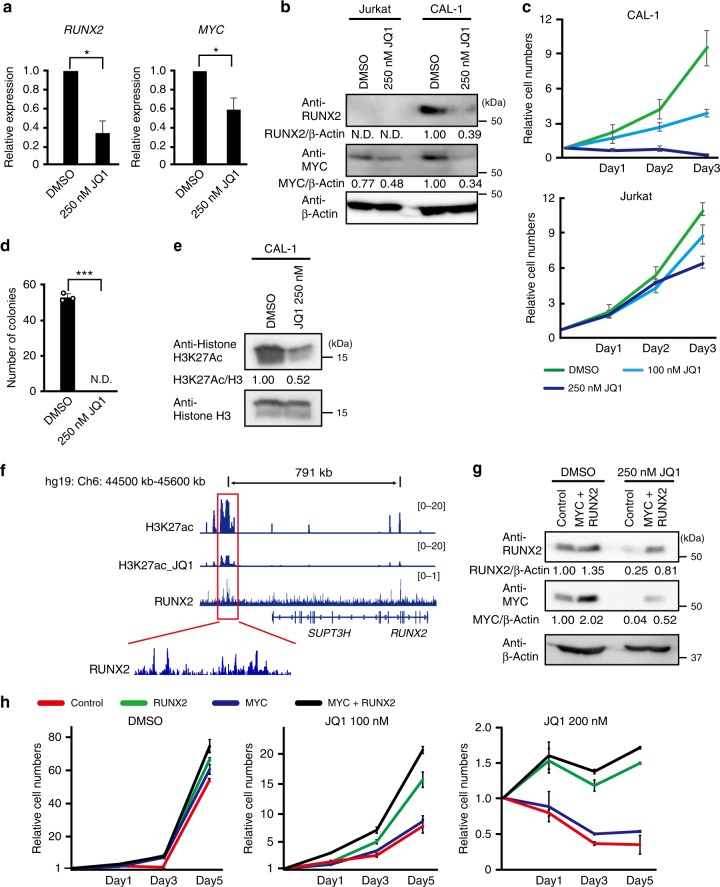
Fig. 4.
MYC and RUNX2 promote the formation of BPDCN by activating the function of enhancers. a Expression levels of RUNX2 and MYC mRNA examined by q-PCR in CAL-1 cells 1 day after the 250 nM JQ1 treatment (n = 3). b CAL-1 cells showing reduced levels of the RUNX2 and MYC proteins 1 day after the 250 nM JQ1 treatment. Levels of β-Actin were used as loading controls. c Decreased cell growth capacities in CAL-1 cells after the JQ1 treatment at 100 nM (light blue line) and 250 nM (blue line) from that in cells treated with DMSO (green line), but not in Jurkat cells (n = 3). d Impaired colony formation capacities of CAL-1 cells treated with 250 nM JQ1 from that in cells treated with DMSO (n = 3). e Decreased level of H3K27ac expression in CAL-1 cells 1 day after the 250 nM JQ1 treatment. f RUNX2 binding to the super-enhancer of RUNX2 that reduced level of H3K27ac post JQ1 treatment in CAL-1 cells assessed by H3K27ac- and RUNX2-ChIP sequencing. g Successful transduction of RUNX2 and MYC in CAL-1 cells with or without the JQ1 treatment at 250 nM examined by western blotting. Levels of β-Actin were used as loading controls. h RUNX2 alone and RUNX2 plus MYC transductions restoring the impaired cell growth post JQ1 treatment. Empty controls (red lines), RUNX2 alone (green lines), MYC alone (blue lines), and RUNX2 plus MYC vectors (black lines) were transduced in CAL-1 cells, which were then treated with JQ1 at 100 nM and 200 nM under liquid culture conditions (n = 3). a, c, d, h Bars show the mean±SD, *p < 0.05 and ***p < 0.001 by the Student’s t-test. Data are representative of 2–3 independent experiments