Abstract
Osteoarthritis (OA) is the most common joint disorder. The Osteoarthritis Initiative (OAI) is a multicentre, longitudinal, prospective observational cohort study of knee OA that aims to provide publicly accessible clinical datasets, images and biospecimens, to enable researchers to investigate factors that influence the onset and development of OA, and evaluate biomarkers that predict and track the course of the disease. In this Perspectives, we describe the rationale and design of the OAI and its cohort, discuss imaging protocols and summarize image analyses completed to date. We include descriptive analyses of publicly available longitudinal (2-year) data of changes in cartilage thickness in a core sample of 600 knees from 590 participants in the OAI progression subcohort. Furthermore, we describe published methodological and applied imaging research that has emerged from OAI pilot studies and OAI data releases, and how these studies might contribute to clinical development of biomarkers for assessing the efficacy of intervention trials.
Introduction
The Osteoarthritis Initiative (OAI)1 is an ongoing multicentre observational cohort study of knee osteoarthritis (OA), jointly sponsored by the NIH and the pharmaceutical industry. Targeted at identifying sensitive imaging and biochemical biomarkers for the onset and progression of knee OA, and for use in evaluating the effectiveness of disease-modifying therapy, the OAI has enrolled almost 4,800 participants with, or at risk of developing, symptomatic knee OA. To date, clinical, radiographic, and MRI data from baseline and 4 years of annual follow-up have been made publicly available to the research community.1
Publications using OAI data have examined the precision, sensitivity to change, and correlation with clinical covariates of imaging outcomes, including radiographic joint-space width (JSW), cartilage thickness, cartilage composition, meniscus morphology, and muscle cross-sectional areas. In this article and the accompanying supplementary information online, we provide a comprehensive overview of the ongoing achievements of the OAI.
Rationale for and design of the OAI
OA is one of the most common diseases of mankind and the most common synovial joint disorder. Owing to its high prevalence and impact on physical function, OA is one of the most frequent causes of disability in developed nations. The number of Americans with clinical (symptomatic) OA (27 million in 2005) is expected to increase dramatically as the population ages. In 2004, half a million primary knee replacement procedures were performed for OA in the USA, and by 2030 that number is projected to grow to 3.5 million. Therefore, a better understanding of the aetiology and risk factors of the disease, and validated imaging biomarkers that can be used to test the efficacy of novel disease-modifying treatments that postpone or prevent the need for joint replacement, are urgently needed. Current drug therapies for OA target symptoms but not the cause of the disease, and no treatments currently approved by regulatory agencies are able to inhibit the progression of structural changes that underlie symptoms. Clinical testing of new pharmacological and nonpharmacological therapies is complicated by the slow development and highly variable manifestation of OA.
Radiography has long been the primary means of assessing structural change, but is limited by low sensitivity to disease progression and by inability to image nonosseous pathology. Whereas OA has traditionally been considered a disease of articular cartilage, its pathogenesis is now accepted to encompass all articular tissues. Essential steps to enable progress in combating OA include a better understanding of the risk factors for OA, a comprehensive characterization of its natural history in all articular tissues, and the development of sensitive biomarkers for testing effectiveness of treatments. For these aims, longitudinal state-of-the-art imaging studies are needed in large, well-characterized populations of persons with, or at risk of developing, OA over a period of time in which clinical change can be clearly defined.
To address these challenges, the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) organized a series of meetings and workshops in 1999, to plan the OAI.1 The outcome was the creation of a multicentre, longitudinal, prospective observational cohort study of knee OA that also includes imaging of hip and hand OA. The overarching goal is to provide publicly accessible clinical datasets, radio-graphs, magnetic resonance imaging (MRI) data and biospecimens to enable researchers worldwide to describe the full natural history of OA, investigate factors that influence disease onset and development, and evaluate biomarkers that predict and track its course. Open access to the data and images has the potential to increase the generation of new knowledge from the study and to accelerate our understanding of the disease through an international collaborative effort. The OAI differs from other large-cohort OA studies in being specifically designed to contribute to the clinical development and qualification of imaging, biochemical and genetic OA biomarkers. It is the only such study to use standardized 3 Tesla MRI (3T-MRI) scanners and protocols, and to make all data available for public use. 3T-MRI rather than 1.5T-MRI was selected on the basis of greater signal-to-noise and contrast-to-noise ratios that can be achieved using the greater field strength provided by the former, which potentially translates into greater precision and sensitivity to change. Furthermore, a comprehensive MRI protocol was implemented,2 to permit determination of a wide variety of quantitative and semiquantitative imaging biomarkers. This step was taken to enable exploration of the most promising markers in the contexts of predicting the onset of symptomatic knee OA, predicting its (structural) progression, and predicting clinical outcomes.
Almost 100 published papers have emerged to date from clinical and imaging data in the OAI cohort (Table 1). The first original paper to use OAI pilot study data was published in 2006, with a sharp increase in the rate of publications in 2009 after the first large-scale data releases had become available. 21 abstracts from the OAI were presented at the World Congress of Osteoarthritis 2011 (18 with imaging data), and 37 in 2012 (27 with imaging data). Eventually, imaging data from the entire cohort is expected to be analyzed and published, but given the substantial amount of resources and funding required, this process will take some time; to date, images of only subsets of the OAI cohort have been studied.
Table 1 |.
Number of OAI publications*
| Year | Number of publications | Number of imaging publications |
|---|---|---|
| 2006 | 1 | 1 |
| 2007 | 3 | 3 |
| 2008 | 3 | 2 |
| 2009 | 15 | 12 |
| 2010 | 28 | 22 |
| 2011 | 27 | 17 |
| 2012‡ | 22 | 16 |
| Total | 98 | 73 |
According to publications listed on the OAI website;
excludes reviews, overviews and study design papers.
Last updated 30 May 2012.
To provide a comprehensive and systematic summary of literature that has emerged from OAI imaging data, we reviewed all original papers that used OAI (or OAI pilot study) images and that are listed on the OAI website,1 excluding reviews, overviews, and study design papers. In addition, we performed a systematic search of the PubMed database in February 2012, using the key words “Osteoarthritis Initiative” and “OAI”. Further literature was included during the revision in May 2012. To com ply with editorial limits that apply to Perspectives articles, OAI imaging literature of clinical relevance is discussed in the main text, whereas more technically-oriented papers have been included in Supplementary Tables 1–3 online.
The OAI cohort
Starting in 2002, the four OAI clinical centres (all located in the USA) enrolled approximately 4,800 male or female participants of all ethnicities, aged 45–79 years, with, or at high risk of, knee OA incidence or progression (Box 1). The sample size was based on the statistical power needed to determine whether biomarkers predict relatively uncommon endpoints, such as the onset (incidence) of symptomatic knee OA or joint replacement, and to compare correlations of changes in different bio markers over time with continuous measures of progression of symptoms and/or joint-space loss and cartilage loss. Such studies can be performed using nested case–control or case–cohort analytical designs, in which subsets of knees with and without clinical or structural outcomes of interest are compared. The overall sample size is also large enough to accommodate studies of other informative subsets of subjects (that is, those with unilateral pain or unilateral radio-graphic OA) while still having adequate numbers for analysis.
Box 1 |. OAI subcohort assignment at baseline.
Upon enrollment, participants in the OAI were assigned to one of three subcohorts. The subcohort distributions of KLGs provided in this Box reflect centralized readings that replace readings made at clinical centres at baseline during recruitment.*
Progression subcohort (n = 1,390)
Comprises individuals with prevalent symptomatic femorotibial OA in one or both knees; furthermore, in any knee with frequent pain (pain on most days of a month in the past year) definite femorotibial osteophytes were present in the same knee. KLG distribution: KLG0, n = 347; KLG1, n = 338; KLG2, n = 920; KLG3, n = 719; KLG4, n = 213; knees without reading results (for example, those with a prosthesis), n = 243.
Incidence subcohort (n = 3,284)
Comprises individuals at risk of development of symptomatic femorotibial OA; patients had one or more of the following risk factors for knee OA:
frequent knee pain in a knee without radiographic OA
radiographic OA without frequent pain
infrequent knee pain
overweight for age and gender
history of knee injury/surgery
family history of knee replacement
Heberden’s nodes
age 70–79 years
Furthermore, in any knee with frequent pain, no radiographic femorotibial OA was present. KLG distribution: KLG0, n = 2,882; KLG1, n = 1,228; KLG2, n = 1,435; KLG3, n = 719; KLG4, n = 213; knees without reading results, n = 424.
Non-exposed reference cohort (n = 122)
Individuals without OA (no symptoms in either knee, no radiographic femorotibial OA in either knee), and with no risk factors for knee OA. KLG distribution: KLG0, n = 213; KLG1, n = 23; KLG2, n = 4; knees without reading results, n = 4.
*Thus, the distribution of KLGs here differs to that from when the cohorts were first assigned. Abbreviations: KLG, Kellgren–Lawrence grade; OA, osteoarthritis; OAI, OA Initiative.
Exclusion criteria for the OAI cohort were bilateral end-stage knee OA—knee arthroplasty or bilateral radiographic Kellgren–Lawrence grade (KLG) 4, the maximum on the 0–4 KLG scale—and inflammatory arthritis. Located at the University of Maryland School of Medicine and the Johns Hopkins University (Baltimore, MD), the Ohio State University (Columbus, OH), the University of Pittsburgh (Pittsburgh, PA), and the Memorial Hospital of Rhode Island (Pawtucket, RI), the clinical centres have now followed the cohort for up to 8 years. Participants are assessed annually in examinations and interviews using both traditional outcome measures (radiography, joint pain, disability, joint replacement), and novel endpoints derived from MRI findings and biochemical biomarkers. At enrollment, participants were assigned to one of three subcohorts, representing the full spectrum of disease development and progression, on the basis of a local (site) reading of the baseline knee radiograph and the presence of knee symptoms and risk factors for knee OA (Box 1).
The OAI MRI protocol
As mentioned above, the architects of the OAI opted to use 3T-MRI systems because of the advantages they offer over lower field-strength scanners in terms of signal-to-noise and contrast-to-noise ratios, spatial resolution, and/or acquisition time. MRI scanners used by the OAI undergo monthly quality assurance testing (Supplementary Table 1 online). The OAI MRI protocol was designed to support a thorough clinical and research evaluation of the femorotibial and patellofemoral joints of both knees, to support as broad a range of existing and anticipated measurement methods for as many articular structures and features as possible (Figure 1), while keeping the total scan time within a range tolerated by most participants (60 min).2 Because of the relative lack of experience with 3T-MRI systems, pilot testing of novel sequences was undertaken.3 The core knee MRI protocol of the OAI requires 60 min of acquisition time2 and was designed for assessment of both quantitative and qualitative measures of OA pathology (the OAI imaging protocol is outlined in Table 2).
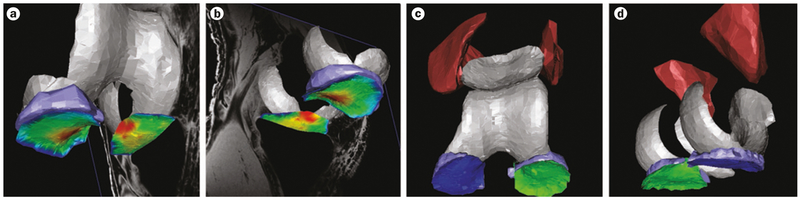
Figure 1 |.
3D rendering of the knee cartilage, meniscus, and thigh muscles in an OAI participant, from MRI data. a | Cartilage thickness in the medial and lateral tibia is displayed by false colours; red denotes thick and dark blue indicates very thin cartilage; the meniscus is displayed in light blue; view from anterior (inferior and lateral). b | Colours are displayed as in part a; viewed from posterior (inferior and lateral). c | Tibial cartilage plates are displayed in dark blue (medial) and green (lateral); the menisci are shown in light blue; the quadriceps (medial and lateral vastus) are shown in dark red; viewed from anterior (inferior and lateral). d | Colours displayed as in part c; viewed from posterior (inferior and medial). Abbreviation: OAI, Osteoarthritis Initiative.
Table 2 |.
OAI MRI protocol and uses for each image acquisition technique
| Imaging technique | Spatial resolution: slice thickness/in-plane area (mm) | Knees examined | Primary purpose(s) (secondary purpose) |
|---|---|---|---|
| Radiography | |||
| Fixed flexion radiography | NA | Both | KLG, osteophyte and JSN grades, quantitative JSW analysis |
| Full limb radiography | NA | Both | Alignment analysis |
| MRI of the knee | |||
| Coronal intermediate-weighted 2D TSE | 3.0/0.37 × 0.46 | Both | Semiquantitative tissue scoring* |
| Sagittal intermediate-weighted 2D TSE with fat suppression | 3.0/0.37 × 0.51 | Both | Semiquantitative tissue scoring* |
| Sagittal 3D DESS water excitation | 0.7/0.37 × 0.46 | Both | Quantitative cartilage analysis (semiquantitative tissue scoring*) |
| Coronal T1W 3D FLASH water excitation | 1.5/0.31 × 0.31 | Right | Quantitative cartilage analysis (semiquantitative tissue scoring*) |
| Sagittal 2D MESE | 3.0/0.31 × 0.45 | Both | Quantitative T2 analysis‡ |
| Axial T1W 2D spin echo (thigh) | 5.0/0.98 × 0.98 | Right | Quantitative muscle analysis |
For example, as assessed by WORMS, BLOKS or MOAKS.
Analysis of T2 relaxation times of cartilage. Abbreviations: BLOKS, Boston Leeds OA knee score; DESS, double echo at steady state; FLASH, fast low angle shot; KLG, Kellgren–Lawrence grade; JSN, joint-space narrowing; JSW, joint-space width; MESE, multi echo spin echo; MOAKS, MRI OA knee score; NA, not applicable; OA, osteoarthritis; OAI, OA Initiative; TSE, turbo spin echo; T1W, T1 relaxation time weighted; WORMS, whole organ MRI score.
Publicly available image data
Clinical data, joint and muscle imaging data, and readings or measurements from these OAI images are available online1 and are summarized in Table 3. Knee radiographs from the first five annual examinations for all participants for whom follow-up images are available have been read centrally for KLGs and individual radiographic features, and, for the majority, radiographic JSW has been measured quantitatively. MRI-based quantitative assessments of cartilage thickness change are also available; 2-year longitudinal data from a core sample of the progression subcohort (600 knees of 590 OAI participants) are summarized in Table 4, which provides the mean change, and variability thereof, in different regions of the joint (according to the online data). The greatest rate of change was observed in the central part of the weight-bearing medial femoral condyle (−5.0% over 2 years) and in the external medial tibia (−5.1%); the greatest sensitivity to change (calculated as standardized response mean [SRM], which is mean change divided by the standard deviation of change across all knees) was observed in the total femorotibial compartment (−0.56), followed by the central medial femorotibial compartment (−0.51). In other words, these regions have the most beneficial ratio between magnitude of change and the variability of the change between participants, and are thus the regions most ‘sensitive’ to change. Image analysis is ongoing in several large projects (funded, for example, by ancillary grants from the NIH) and more image analysis data will hence become publically available in the future.
Table 3 |.
OAI image analysis completed to date* and available for public use1
| Imaging technique | Measurements | Approximate sample size (knees) | Timepoints |
|---|---|---|---|
| Knee radiography | |||
| Fixed flexion radiography | KLG, individual features (OARSI grades) | 4,200 (8,400) | 0, 12, 24, 36, and 48 months |
| Fixed flexion radiography | Quantitative JSW (minimum, fixed location) | 2,835 (5,095) | 0, 12, 24, 36, and 48 months |
| Knee MRI | |||
| Various sequences | Femorotibial cartilage volume, thickness, etc. | 702 (732) | 0, 12, and 24 months‡ |
| Sagittal DESS | Femorotibial cartilage volume, thickness, etc. | 590 (600) | 0, 12, and 24 months‡ |
| Sagittal DESS | Femorotibial cartilage volume, thickness, etc. | 85 (86); subset of sample above | 0, 12, 24, and 48 months‡ |
Image analysis is ongoing in several larger projects (for example, in ancillary studies) and more data will be released in the future.
Some knees do not have 12-month follow-up data, but all have 24-month follow-up data. Abbreviations: DESS, double echo at steady state; JSW, joint-space width; KLG, Kellgren–Lawrence grade; OA, osteoarthritis; OAI, OA Initiative; OARSI, OA Research Society International.
Table 4 |.
| Region | Baseline to 12 months (565 knees) | Baseline to 24 months (600 knees) | ||
|---|---|---|---|---|
| Mean change (%) | SRM§ | Mean change (%) | SRM§ | |
| Compartments | ||||
| Total femorotibial | −1.30 | −0.44 | −2.20 | −0.56 |
| Medial femorotibial | −1.60 | −0.35 | −2.60 | −0.44 |
| Lateral femorotibial | −1.10 | −0.32 | −1.70 | −0.37 |
| Central medial femorotibial | −2.60 | −0.43 | −4.10 | −0.51 |
| Central lateral femorotibial | −1.30 | −0.35 | −2.10 | −0.40 |
| Cartilage plates | ||||
| Medial tibia | −1.00 | −0.23 | −1.80 | −0.34 |
| Medial weight-bearing femoral condyle | −2.10 | −0.33 | −3.40 | −0.41 |
| Lateral tibia | −1.30 | −0.35 | −2.40 | −0.48 |
| Lateral weight-bearing femoral condyle | −0.90 | −0.19 | −1.10 | −0.19 |
| Medial femorotibial subregions | ||||
| Central medial tibia | −2.10 | −0.32 | −3.40 | −0.43 |
| External medial tibia | −2.90 | −0.28 | −5.10 | −0.36 |
| Internal medial tibia | 0.20 | 0.04 | −0.10 | −0.02 |
| Anterior medial tibia | −0.30 | −0.05 | −0.70 | −0.12 |
| Posterior medial tibia | −0.40 | −0.08 | −0.60 | −0.09 |
| Central medial weight-bearing femoral condyle | −3.20 | −0.35 | −5.00 | −0.44 |
| External medial weight-bearing femoral condyle | −1.50 | −0.16 | −2.80 | −0.24 |
| Internal medial weight-bearing femoral condyle | −1.60 | −0.27 | −2.30 | −0.33 |
| Lateral femorotibial subregions | ||||
| Central lateral tibia | −1.50 | −0.32 | −2.90 | −0.44 |
| External lateral tibia | −1.20 | −0.19 | −2.10 | −0.27 |
| Internal lateral tibia | −1.80 | −0.26 | −3.40 | −0.44 |
| Anterior lateral tibia | −0.50 | −0.10 | −0.60 | −0.11 |
| Posterior lateral tibia | −1.40 | −0.26 | −2.60 | −0.38 |
| Central lateral weight-bearing femoral condyle | −1.00 | −0.20 | −1.20 | −0.19 |
| External lateral weight-bearing femoral condyle | −1.00 | −0.13 | −1.10 | −0.11 |
| Internal lateral weight-bearing femoral condyle | −0.30 | −0.06 | −1.00 | −0.15 |
The greatest rate of change is observed in the central part of the weight-bearing medial femoral condyle and in the external medial tibia (see Figure 4 for cartilage regions); the greatest sensitivity to change (SRM) is observed in the total femorotibial compartment followed by the central medial femorotibial compartment.
Obtained with a sagittal double echo steady state MRI sequence in a core sample of the OAI progression subcohort and available online.1
SRM calculated as mean change divided by standard deviation of the change across all knees. Abbreviations: OAI, Osteoarthritis Initiative; SRM, standardized response mean.
Pilot and methodological studies
The MRI scanners at the four OAI centres are monitored using phantoms (which check consistency of images produced) to ensure that longitudinal measurements are not affected by scanner drift. Consistency in terms of MRI findings between the four OAI centres has been demonstrated, alongside good signal and geometric stability over the first 3 years’ follow-up (Supplementary Table 1 online).
OAI MRI pilot studies
A pilot study was conducted to evaluate the test–retest precision of quantitative cartilage analysis, and its sensitivity to changes in cartilage thickness over 1 and 2 years, using images from different 3T-MRI sequences included in the OAI acquisition protocol. Performance metrics were compared between four groups conducting the image analysis,3 and between different MRI contrasts—for example FLASH, which is T1-weighted, and double echo at steady state (DESS), which involves both T1-weighted and T2-weighted contrast—and have also been published in part by individual groups (Supplementary Table 1 online). The four-group comparison3 concluded that cartilage morphology metrics, such as cartilage volume or thickness obtained using different image contrasts in the same knees had similar test–retest precision and were generally equivalent: data from different contrasts can therefore be combined. Given systematic differences in results from different analysis teams, it was concluded that data from different teams should not be pooled unless equivalence is demonstrated for the metric of interest in future cross-calibration studies.3 In other papers, FLASH and DESS acquisitions obtained with different MRI knee coils (phased-array and quadrature coils) were compared and found to involve slight differences (offsets) in cartilage volume and thickness measures obtained. Two groups also reported longitudinal (2-year follow-up) results from OAI pilot studies (Supplementary Table 1 online) and concluded that FLASH and DESS were sensitive to change in cartilage volume and thickness seen in knee OA.
OAI methodological studies
Several methodological comparisons have been made regarding quantitative radio graphic data generated by the OAI (Supplementary Table 2 online). The findings emphasize, for example, the need to take radio-anatomic alignment of OAI fixed-flexion radiographs into account when analyzing change in JSW, and the need for central radiographic readings. Regarding semiquantitative scoring of articular tissue pathology using MRI images, two existing systems—WORMS (whole organ MRI score) and BLOKS (Boston Leeds osteoarthritis knee score)—were applied to a sample of images of 113 knees with radiographic OA and at risk of progression, from the OAI cohort. Both methods were shown to be reliable cross-sectionally (Supplementary Table 2 online). Longitudinally, BLOKS was found to be superior to WORMS for assessment of change in the meniscus, and WORMS was superior to BLOKS for scoring bone-marrow lesions (BMLs), in terms of predicting cartilage loss.4 A new hybrid method (MOAKS; MRI OA knee score) was hence proposed with the aim of combining the advantages of each scoring system.5 In assessing which sequence is better to detect such changes, more and larger focal cartilage defects and BMLs were detected with the intermediate-weighted fat-suppressed spin echo sequence than with DESS6,7 (Figure 2, Supplementary Table 2 online).
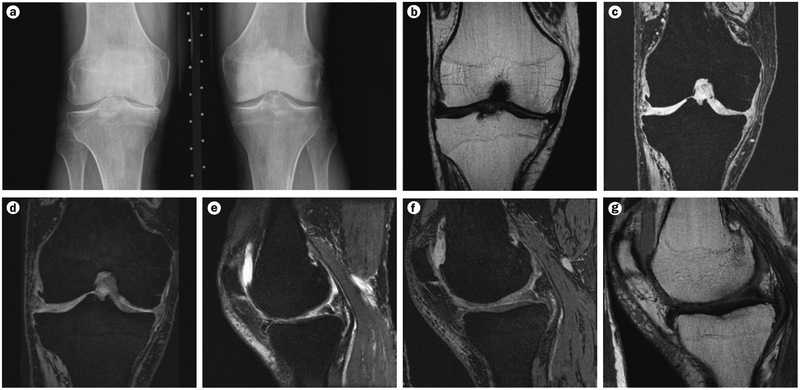
Figure 2 |.
Sample images generated using the OAI knee imaging protocol. a | Anteroposterior fixed flexion radiograph. b | Coronal intermediate-weighted 2D turbo spin echo MRI image. c | Coronal T1-weighted 3D fast low angle shot with water excitation MRI image. d | Coronal multiplanar reconstruction of the sagittal double echo steady state acquisition shown in part e. e | Sagittal 3D double echo steady state acquisition with water excitation MRI image. f | Sagittal intermediate-weighted 2D turbo spin echo with fat-suppression MRI image. g | Sagittal 2D multi-echo spin echo (echo time 10–70 ms; the MRI image displayed shows the acquisition with 10 ms echo time). Abbreviation: OAI, Osteoarthritis Initiative.
Semi-automated segmentation algorithms for quantitative measurement of cartilage, bone, meniscus, and thigh muscles (Figure 3) have been assessed. These studies have used different image analysis approaches and have reported, in part, the level of agreement with manual segmentation and/or the level of inter-observer reliability (Figure 1, Supplementary Table 2 online).
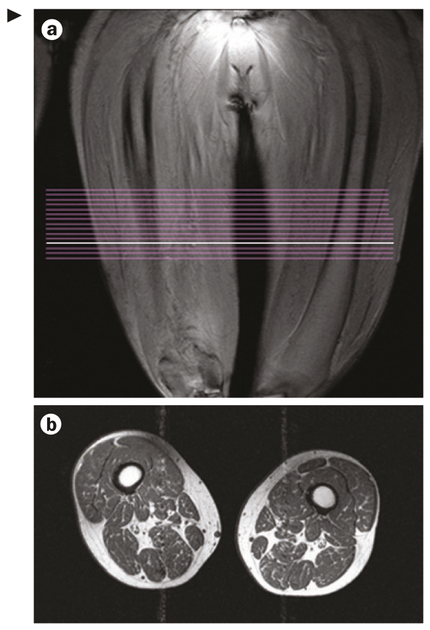
Figure 3 |.
Sample images generated using the OAI thigh imaging protocol. a | Image from coronal localizer MRI used to delineate the distal femoral epiphysis and to position a slab of 15 axial contiguous slices with 5 mm thickness. Acquisition starts 10 cm proximal to the distal femoral epiphysis and extends 7.5 cm proximally. Note that owing to the fixed distance (10 cm) between the distal femoral epiphysis and the most distal image acquired, as per OAI protocol, the position of the images relative to the length of the femur and thigh muscles of the participants vary depending on femoral length and body height. Comparisons between participants should therefore take this variability into account by selecting anatomically corresponding images, by adjusting for femoral length or body height. The white line indicates the location of the acquired images shown in part b. b | Axial T1-weighed 2D spin echo of the thighs. Abbreviation: OAI, Osteoarthritis Initiative.
The sensitivity to change of cartilage thick ness over 1 year in the medial femorotibial compartment was found to be similar between sagittal DESS, coronal multiplanar reconstructed DESS, and coronal FLASH in 80 knees (Figure 2), with SRMs ranging from −0.34 to −0.38.8 The three protocols were also highly intercorrelated cross-sectionally (coefficient of correlation [r] ≥0.94); analysis of every second 0.7 mm DESS image provided similar sensitivity to change as analysis of every image.8 Change in the medial weight-bearing femur substantially exceeded that in the posterior aspect of the femoral condyle, suggesting that structural progression is faster in (commonly) weight-bearing regions of the joint.9
Measuring between-group differences using cartilage subregions (Figure 4) or atlases of cartilage thickness within anatomically defined cartilage plates has also been explored by several groups, alongside assessing whether such methods improve sensitivity to change (Supplementary Table 3 online). These studies generally identified the central subregion of the weight-bearing medial femoral cartilage plate as the region of interest with the greatest rate of cartilage loss and sensitivity to change (Figure 4).
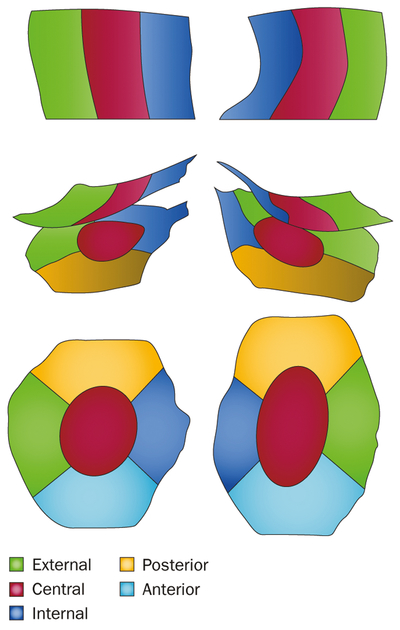
Figure 4 |.
Regions and subregions of the knee that are frequently used to track changes in cartilage thickness. Subregions of cartilage are distinguished by colour. The top part of the figure shows the weight-bearing part of the femoral condyles (central medial and central lateral), as viewed from inferior. The middle part shows the weight-bearing femorotibial cartilage region, as viewed from posterior. The bottom part shows medial and lateral tibiae, as viewed from superior.
First-release image analysis
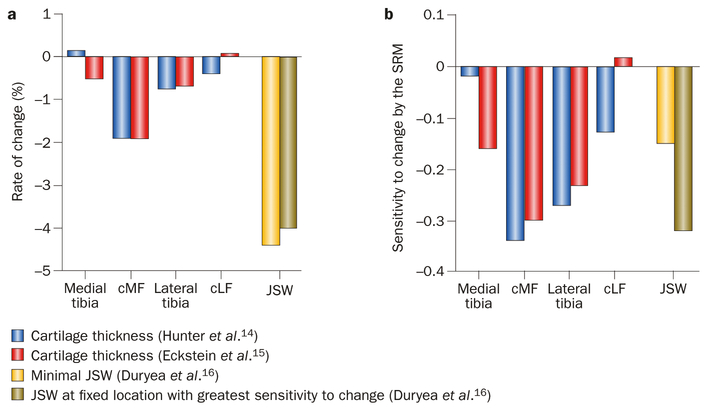
Analyses of the first public release of OAI knee images—from 160 participants with symptomatic knee OA—showed that meniscal damage was common (79% medially, 39% laterally) and was associated with presence of BMLs in the same (medial or lateral) joint compartment.10 BML and joint effusion scores (using BLOKS), but not synovitis, were independently associated with knee pain (using the Western Ontario and McMaster Universities Osteoarthritis Index; WOMAC), suggesting that pain in knee OA has more than one potential source.11 14% of the participants had an anterior cruciate ligament (ACL) tear, and had more denuded areas (exposure of subchondral bone), meniscal damage, and BMLs than those without tears.12 Furthermore, ACL tears were related to femoral notch stenosis.13 1-year cartilage volume loss rates in symptomatic knees (reported using semi-automated segmentation of DESS)14 and cartilage thick ness loss in all right knees of this sample (using manual segmentation of FLASH images)15 were similar between both contrasts across the four femorotibial cartilage plates (Figure 5a), with relatively modest SRMs (Figure 5b). Use of fixed flexion radiography at an optimal fixed location (0.275) was more sensitive to change in JSW than assessing the point of minimal JSW, using automated software16 (Figure 5). No significant differences in cartilage thickness loss by sex, BMI, symptom status, or KLG were observed in this sample,15 but SRMs were substantially greater in knees with denuded subchondral bone areas,17 JSN (but not osteophytes), subchondral bone sclerosis, and low cartilage thickness at baseline,18 suggesting that knees at advanced stages of structural joint disease have greater structural progression than those at earlier stages.
Figure 5 |.
Longitudinal (1-year) loss of cartilage in different regions of the knee (MRI data) and of medial radiographic JSW. The data come from the first release of data by the OAI: a subsample of the OAI progression subcohort (n = 160), stratified by age and sex, and analyses by Hunter et al.,14 Eckstein et al.,15 and Duryea et al.16 a | Rates of change in cartilage and JSW. b | Sensitivity to change as expressed by the SRM.* Hunter et al.14 and Duryea et al.16 examined knees with frequent symptoms and definite radiographic knee OA in 150 participants of this cohort using the sagittal double echo steady state MRI sequence and reported normalized cartilage volume loss (trimmed at joint margins); Eckstein et al.15 examined 157 right knees of this cohort using the coronal fast low angle shot MRI sequence and reported cartilage thickness loss. SRMs show the same pattern throughout the cartilage plates in both studies, with the femur (cMF) showing greater changes (and sensitivity) than the tibia medially, and the tibia showing greater changes (and sensitivity) than the femur (cLF) laterally. *Mean change divided by the standard deviation of the change. Abbreviations: cLF, lateral weight-bearing (central) femoral condyle; cMF, medial weight-bearing (central) femoral condyle; JSW, joint-space width; OA, osteoarthritis; OAI, OA Initiative; SRM, standardized response mean.
Large-release OAI imaging data
Cartilage thickness analysis
An aim of the OAI is to relate cartilage thickness changes to radiographic disease status. In the non-exposed reference cohort of the OAI (Box 1), subregional cartilage thickness was similar to that in a large population-based cohort without OA.19 A cross-sectional study in >1,000 participants representing all KLGs reported significantly larger subchondral bone areas in OA than in reference cohort knees (~10% larger, P <0.01).20 Compared with reference knees, cartilage thickness differences were greatest in the external medial tibia of OA knees with medial JSN, and in the external lateral tibia of those with lateral JSN.20 Greater cartilage thickness than in healthy reference participants was reported in the external central medial femur of knees with early radiographic OA (KLG ≤2),20 and confirmed in the external weight-bearing medial (and lateral) femur in a study that compared knees with osteophytes (and without JSN) with contralateral knees without osteophytes or JSN, using a between-knee, within-person design.21 Although this combination of radiographic features was observed in only 1.3% of the 4,796 OAI participants, the OAI cohort size enables such analyses, which exclude confounding from between-person differences.
Denuded areas were present at early stages of radiographic OA (KLG1 and KLG2) and became more common (and larger) with increasing radiographic disease severity.22 In 73 participants with medial Osteoarthritis Research Society International (OARSI) JSN grade 1–3 in one knee and a contralateral knee without JSN, medial JSN grades 1–3 were associated, respectively, with 5%, 18%, and 44% reductions in medial femorotibial cartilage thickness.23
Longitudinal (1-year) analysis of change in cartilage thickness in 719 knees with radiographic OA found a low rate of change in KLG2 knees (≤1%, SRM up to −0.22; not significantly different from 112 reference cohort knees), up to −2.5% (SRM up to −0.35) in KLG3, and up to −3.9% (SRM up to −0.51) in KLG4.24 In the same sample, an extended ordered value approach (which ranks subregional femorotibial cartilage loss in the medial and lateral compartments based on its magnitude, and computes averages across these ranks—or ordered values—rather than across specific locations) was superior in differentiating rates of progression between KLG3 and KLG2 knees (P = 5.4 × 10−7) to the region-based approach (P = 0.008) and radiography (P = 0.386).25 Furthermore, in the same sample it was found that knees with frequent pain displayed greater rates of cartilage loss than those without pain, even after adjustment for or stratifying by KLG.26 Using a between-knee, within-person design, MRI-based cartilage loss rates were greater in knees with JSN than those without (both painful),9 whereas radiographic JSW change did not differ between the knees, but was more variable in knees without JSN.27 Radiographic JSN in hand radiographs from this cohort was associated with reduced knee cartilage thickness, and hand osteophytes with radio-graphic knee OA status, but neither was associated with structural progression in the knee.28 Systemic susceptibility to OA, and possibly different mechanisms for osteophyte formation and cartilage thinning, are suggested by these findings.28 Over 2 years, the SRM for cartilage thickness change in 346 OAI participants was modestly higher than over 1 year,29 and the rate of change was not significantly different between the first and second year.29 In summary, these data suggest that when MRI-based cartilage thickness outcomes are used as measures of progression, structural disease progression is more likey to occur in painful knees with advanced radiographic OA status than knees without pain and without radiographic JSN.
Cartilage lesions and composition
In asymptomatic, middle-aged incidence cohort participants, cartilage lesions (including signal changes without focal loss) were found in 75%, meniscal lesions in 47%, and BMLs in 40% of knees studied,30 with a similar prevalence of lesions reported in the non-exposed reference cohort.31 These lesions were more frequent in subjects with greater levels of physical activity,30 greater BMI,32 and ACL injuries.33 Over 36 months, the incidence and progression of cartilage lesions (but not meniscus lesions) was greater in obese than in non-obese OAI participants.32
Cartilage T2 (relaxation times) are thought to be sensitive to cartilage hydration, collagen content, and tissue anisotropy and thus represent a quantitative mea sure of cartilage composition.31,33,34 Femorotibial and patellar cartilage T2 were greater in asymptomatic incidence cohort knees that had cartilage and meniscus lesions (as assessed by WORMS) than in those without lesions.35 Patellar34 and femorotibial36 cartilage T2 were associated with greater physical activity scores and frequent knee-bending activity in participants both with and without risk factors for knee OA. Cartilage T2 was higher37,38 and more heterogeneous38 in knees of individuals with OA risk factors than in those of reference cohort participants, but did not differ between knees with and without ACL tears.33 Greater cartilage T2 was associated with pain in early OA knees, whereas in knees with morphological abnormalities only cartilage lesions (but not cartilage T2) were associated with pain.39
A significant increase in T2 over 2 years (P = 0.0072) was reported in the femorotibial (but not femoropatellar) cartilage of reference cohort participants, and was associated with progression of cartilage lesions to higher WORMS grades.31 By contrast, neither the presence of OA risk factors nor the presence of cartilage lesions at baseline was associated with the 2-year increase in knee cartilage T2.37 In a 3-year longitudinal study,40 obese patients with risk factors for knee OA had a higher prevalence of cartilage and meniscus lesions at baseline than healthy reference individuals, and a high BMI was associated with progression of cartilage lesions to higher grades and with an increase in cartilage T2 entropy over 36 months. Furthermore, cartilage T2 at baseline was significantly associated with structural worsening of synovial tissues (cartilage, meniscus, bone marrow) over 3 years.41 In summary, these data suggest that progression of cartilage and other tissue lesions, and cartilage T2, are associated with physical activity and OA risk factors (such as obesity) and that T2 might have a relationship with pain and/or structural progression of tissue changes in knee OA.
Meniscus and muscle assessments
Quantitative study of meniscal areas in the healthy reference cohort found ~25% larger tibial plateau and medial meniscus surface areas in men than in women, in knees without meniscus lesions, but the ratio between the two areas, and the tibial plateau coverage by the meniscus (50% medially; 58% laterally) was similar in both sexes.42 Meniscus surface area was more strongly correlated with (ipsi-compartmental) tibial plateau area than with body height or weight, and physiological medial meniscal extrusion (over the tibia) was somewhat greater in healthy women than in men.42 A between-knee, within-person comparison revealed less tibial coverage (−5%) and greater meniscus extrusion (+15%) in frequently painful than in contralateral painless knees of identical KLG.43 In the same sample, significant reductions in quadriceps and vastus medialis muscle anatomical cross-sectional areas (ACSAs) and quadriceps strength were found in painful versus contralateral painless knees, but no differences between painful and painless knees were noted for hamstring and adductor ACSAs, or flexor strength.44 These findings suggest that meniscus extrusion and lack of quadriceps muscle mass and strength are associated with knee pain. Furthermore, the latter findings suggest that quadriceps strengthening exercise might be useful in treating symptomatic knee OA.
In asymptomatic, middle-aged subjects with risk factors for knee OA, a higher ratio of muscle ACSA between the vastus lateralis and medialis was associated with lower knee cartilage T2 and lower presence and severity of synovial tissue changes (as assessed by WORMS). 2-year rates of change in quadriceps ACSAs and intermuscular fat in the OAI incidence cohort were reported to be similar in participants with and without radiographic OA, indicating that loss of muscle mass and strength occur as a function of ageing rather than being associated with radiographic OA.45
Future directions
Prospective, observational studies, such as the OAI, with sufficiently long follow-up to capture slowly-developing OA clinical outcomes are an important resource for bio-marker qualification, the evidentiary process that links biomarkers with clinical endpoints and biology. To gain scientific and regulatory acceptance, biomarkers (both prognostic and for measuring efficacy of intervention) must be characterized in multiple studies for their ability to predict and track clinical outcomes, such as pain, disability, and joint surgery. Observational studies of prognostic imaging biomarkers can directly contribute to qualification of such markers, and can identify potential biomarkers for assessing efficacy of interventions in clinical trials.
Efforts are underway using OAI data to identify relationships between baseline and longitudinal imaging biomarkers and pain worsening or other clinical outcomes. A 2012 study, for instance, reported greater denuded areas and greater longitudinal rates of medial femorotibial cartilage thickness loss in a 1-year observational period prior to knee replacement, compared with control knees without knee replacement that were matched for age and KLG.46 Similar studies focusing on other structural alterations of the diarthrodial joint have been presented at recent conferences. Further efforts are being directed toward defining the need for knee replacement (based on clinical parameters) as a virtual surgical endpoint that can appropriately classify participants who may need, but might be unwilling or unable to undergo, surgery. It is expected that these efforts will identify and appropriately qualify imaging biomarkers within the next 2 years that can be applied in therapeutic trials, to demonstrate usefulness in early decision-making and regulatory approval for showing efficacy of structure-modifying or disease-modifying therapy.
Conclusions
The OAI—involving 4,796 participants with, or at risk for developing, symptomatic knee OA—is an ongoing multi-centre observational cohort study of knee OA jointly sponsored by the NIH and the pharmaceutical industry. It aims to identify sensitive imaging and molecular biomarkers for monitoring the onset and progression of knee OA, and for use in evaluating the effectiveness of disease modifying therapy. Presently clinical, radiographic, and MRI data for baseline, and annual follow-up for 4 years, have been made publicly available to the research community. Publications using the OAI data have examined the precision, sensitivity to change, and correlation with clinical covariates of imaging outcomes, including radiographic JSW, cartilage thickness, cartilage composition, meniscus morphology, and muscle cross-sectional areas. It is expected that through this qualification process, powerful imaging biomarkers will become available within the next 2 years, which can be applied in therapeutic trials in which the efficacy of disease-modifying or structure-modifying OA drugs are evaluated.
Supplementary Material
Acknowledgements
The authors thank the Osteoarthritis Initiative (OAI) investigators, clinical staff and participants at each of the clinical centres and at the coordinating centre for their important contributions in acquiring the publicly available clinical and imaging data. The OAI is a public–private partnership comprising five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the NIH, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer. Private sector funding for the OAI is managed by the Foundation for the NIH.
Footnotes
Competing interests
F. Eckstein declares associations with the following companies: Abbot, Bioclinica, Centocor R&D, Chondrometrics GmbH, Eli Lilly, Genzyme, GlaxoSmithKline, Medtronic, MerckSerono, Novartis, Pfizer, Sanofi-Aventis, Stryker, Synthes, Wyeth. W. Wirth declares associations with the following companies: Chondrometrics GmbH, MerckSerono. See the article online for full details of the relationships. M. C. Nevitt declares no competing interests.
Supplementary information
Supplementary information is linked to the online version of the paper at www.nature.com/nrrheum
References
- 1.Osteoarthritis Initiative Coordinating Centre. OAIOnline [online] http://www.oai.ucsf.edu/(2012). [Google Scholar]
- 2.Peterfy CG, Schneider E & Nevitt M The Osteoarthritis Initiative : report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 16, 1433–1441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider E et al. Equivalence and precision of knee cartilage morphometry between different segmentation teams, cartilage regions, and MR acquisitions. Osteoarthritis Cartilage 10.1016/j.joca.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT et al. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 18, 1402–1407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter DJ et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthritis Cartilage 19, 990–1002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi D et al. Semiquantitative assessment of subchondral bone marrow edema-like lesions and subchondral cysts of the knee at 3T MRI: a comparison between intermediate-weighted fat-suppressed spin echo and dual echo steady state sequences. BMC Musculoskelet. Disord. 12, 198 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roemer FW et al. Semiquantitative assessment of focal cartilage damage at 3T MRI: a comparative study of dual echo at steady state (DESS) and intermediate-weighted (IW) fat suppressed fast spin echo sequences. Eur. J. Radiol 80, e126–e131 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Wirth W et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols—comparative data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage 18, 547–554 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein F et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Rheum. 61, 1218–1225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo GH et al. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 17, 743–747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo GH et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 17, 1562–1569 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein V et al. Pattern of joint damage in persons with knee osteoarthritis and concomitant ACL tears. Rheumatol. Int 32, 1197–1128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein V et al. The relation of femoral notch stenosis to ACL tears in persons with knee osteoarthritis. Osteoarthritis Cartilage 18, 192–199 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter DJ et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann. Rheum. Dis 68, 349–356 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckstein F et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann. Rheum. Dis 68, 674–679 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duryea J et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res. (Hoboken) 62, 932–937 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DJ et al. Region of interest analysis: by selecting regions with denuded areas can we detect greater amounts of change? Osteoarthritis Cartilage 18, 175–183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein F et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the Osteoarthritis Initiative. Arthritis Res. Ther 11 R90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckstein F et al. Reference values and Z-scores for subregional femorotibial cartilage thickness—results from a large population-based sample (Framingham) and comparison with the non-exposed Osteoarthritis Initiative reference cohort. Osteoarthritis Cartilage 18, 1275–1283 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frobell RB et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res. (Hoboken) 62, 1612–1623 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Cotofana S et al. Cartilage thickening in early radiographic human knee osteoarthritis—within-person, between-knee comparison. Arthritis Care Res. (Hoboken) 10.1002/acr.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frobell RB et al. Presence, location, type and size of denuded areas of subchondral bone in the knee as a function of radiographic stage of OA—data from the OA Initiative. Osteoarthritis Cartilage 18, 668–676 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckstein F et al. Magnitude and regional distribution of cartilage loss associated with grades of joint space narrowing in radiographic osteoarthritis—data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage 18, 760–768 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein F et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: results from 831 participants from the Osteoarthritis Initiative. Arthritis Care Res. (Hoboken) 63, 311–319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth W et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography—data from the OA initiative. Osteoarthritis Cartilage 19, 689–699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckstein F et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the Osteoarthritis Initiative. Arthritis Rheum. 63, 2257–2267 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benichou OD et al. One-year change in radiographic joint space width in patients with unilateral joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Care Res. (Hoboken) 62, 924–931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haugen IK et al. Hand joint space narrowing and osteophytes are associated with magnetic resonance imaging-defined knee cartilage thickness and radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. J. Rheumatol 39, 161–166 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Wirth W et al. Comparison of 1-year vs 2-year change in regional cartilage thickness in osteoarthritis results from 346 participants from the Osteoarthritis Initiative. Osteoarthritis Cartilage 19, 74–83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehling C et al. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage 18, 776–786 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology 261, 507–515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laberge MA et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects—data from the Osteoarthritis Initiative. Skeletal Radiol. 41, 633–641 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hovis KK et al. Non-traumatic anterior cruciate ligament abnormalities and their relationship to osteoarthritis using morphological grading and cartilage T2 relaxation times: data from the Osteoarthritis Initiative (OAI). Skeletal Radiol. 10.1007/s00256-012-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stehling C et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the Osteoarthritis Initiative. Radiology 254, 509–520 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan J et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI—an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage 19, 65–73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hovis KK et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 63, 2248–2256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baum T et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the Osteoarthritis Initiative. J. Magn. Reson. Imaging 35, 370–378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph GB et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls—data from the Osteoarthritis Initiative. Arthritis Res. Ther 13, R153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baum T et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res. (Hoboken) 64, 248–255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baum T et al. MRI-based knee cartilage T2 measurements and focal knee lesions correlate with BMI—36 month follow-up data from the Osteoarthritis initiative. Arthritis Care Res. (Hoboken) 10.1002/acr.21741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph GB et al. Baseline mean and heterogeneity of MR cartilage T(2) are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 20, 727–735 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloecker K et al. Size and position of the healthy meniscus, and its correlation with sex, height, weight, and bone area—a cross-sectional study. BMC Musculoskelet. Disord 12, 248 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenger A et al. Relationship of 3D meniscal morphology and position with knee pain in subjects with knee osteoarthritis: a pilot study. Eur. Radiol 22, 211–220 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Sattler M et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 20, 532–540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beattie KA MacIntyre NJ, Ramadan K, Inglis D & Maly MR Longitudinal changes in intermuscular fat volume and quadriceps muscle volume in the thighs of women with knee osteoarthritis. Arthritis Care Res. (Hoboken) 64, 22–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckstein F et al. Quantitative magnetic resonance imaging measures of cartilage predict knee replacement—a case–control study from the Osteoarthritis Initiative. Ann. Rheum. Dis 10.1136/annrheumdis-2011-201164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.