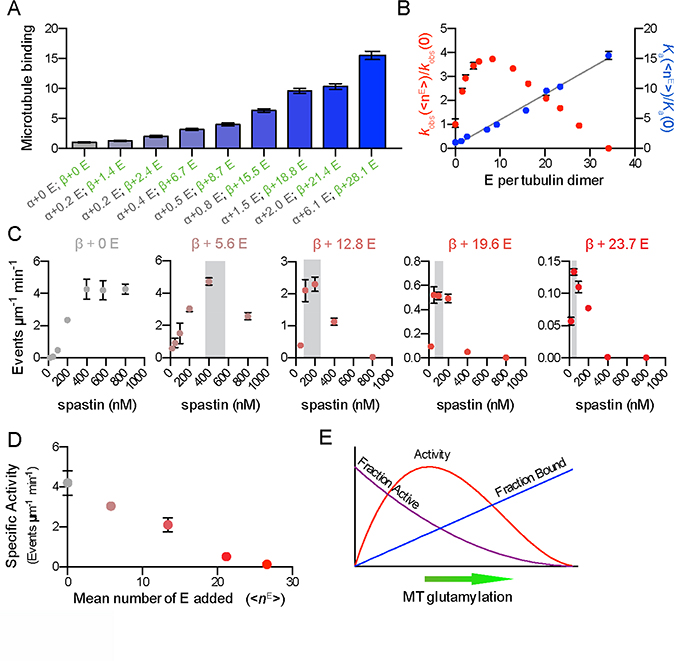
Figure 5. Competition between affinity and specific activity gives rise to the biphasic regulation of spastin-catalyzed microtubule severing by tubulin glutamylation.
(A) Spastin affinity for microtubules increases with the number of glutamates on tubulin tails. Spastin binding on modified microtubules normalized to that for unmodified microtubules). <nE> on α and β-tubulin are indicated as in Figure 1. Error bars, S.E.M. (n = 50 microtubules from multiple chambers for each <nE>).
(B) Spastin microtubule affinity increases linearly with glutamate numbers on tubulin (R2 = 0.99). Left, microtubule severing rates, kobs, right, microtubule association constants, Ka for various <nE> normalized to those for unmodified microtubules. Error bars represent S.E.M.
(C) Microtubule severing rates as a function of spastin concentration for microtubules with various glutamylation levels. Increased glutamylation induces earlier and more abrupt onset of anti-cooperativity of microtubule severing. Error bars, S.E.M. (n > 21 microtubules from multiple chambers for each <nE>). Grey shading, region of transition from cooperative to anti-cooperative behavior.
(D) Microtubule severing activities for different glutamylation levels at constant number of bound spastin molecules per tubulin. The specific activity of bound spastin decreases with increased glutamylation. Error bars, S.E.M. (n > 20 microtubules from multiple chambers for each <nE>).
(E) Competition between glutamylation-induced increase in microtubule affinity and decrease in enzyme specific activity yields a biphasic response of spastin severing to microtubule glutamylation levels. See also Figure S4.