This systematic review and meta-analysis examines randomized clinical trials to assess the safety and efficacy of medications used for patients with gastrointestinal and pancreatic neuroendocrine tumors.
Key Points
Question
What is the available evidence on therapies for neuroendocrine tumors?
Findings
This systematic review and network meta-analysis identified 30 relevant randomized clinical trials comprising 3895 patients with neuroendocrine tumors assigned to 22 different therapies. A network meta-analysis identified 7 therapies for pancreatic neuroendocrine tumors and 5 therapies for gastrointestinal neuroendocrine tumors with a broad range of different toxic effects and higher efficacy than placebo.
Meaning
There appears to be a range of efficient therapies with different safety profiles available for patients with neuroendocrine tumors.
Abstract
Importance
Multiple therapies are currently available for patients with neuroendocrine tumors (NETs), yet many therapies have not been compared head-to-head within randomized clinical trials (RCTs).
Objective
To assess the relative safety and efficacy of therapies for NETs.
Data Sources
PubMed, Embase, the Cochrane Central Register of Controlled Trials, trial registries, meeting abstracts, and reference lists from January 1, 1947, to March 2, 2018, were searched. Key search terms included neuroendocrine tumors, gastrointestinal neoplasms, therapy, and randomized controlled trial.
Study Selection
Randomized clinical trials comparing 2 or more therapies in patients with NETs (primarily gastrointestinal and pancreatic) were evaluated. Thirty RCTs met the selection criteria.
Data Extraction and Synthesis
Pairs of independent reviewers screened studies, extracted data, and assessed the risk of bias. A network meta-analysis with a frequentist approach was used to compare the efficacy of therapies; the Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline was used.
Main Outcomes and Measures
Disease control, progression-free survival, overall survival, adverse events, and quality of life.
Results
The systematic review identified 30 relevant RCTs comprising 3895 patients (48.4% women) assigned to 22 different therapies for NETs. These therapies showed a broad range of risk for serious and nonserious adverse events. The network meta-analyses included 16 RCTs with predominantly a low risk of bias; nevertheless, precision-of-treatment estimates and estimated heterogeneity were limited. The network meta-analysis found 7 therapies for pancreatic NETs: everolimus (hazard ratio [HR], 0.35 [95% CI, 0.28-0.45]), everolimus plus somatostatin analogue (HR, 0.35 [95% CI, 0.25-0.51]), everolimus plus bevacizumab plus somatostatin analogue (HR, 0.44 [95% CI, 0.26-0.75]), interferon (HR, 0.37 [95% CI, 0.16-0.83]), interferon plus somatostatin analogue (HR, 0.31 [95% CI, 0.13-0.71]), somatostatin analogue (HR, 0.46 [95% CI, 0.33-0.66]), and sunitinib (HR, 0.42 [95% CI, 0.26-0.67]), and 5 therapies for gastrointestinal NETs: bevacizumab plus somatostatin analogue (HR, 0.22 [95% CI, 0.05-0.99]), everolimus plus somatostatin analogue (HR, 0.31 [95% CI, 0.11-0.90]), interferon plus somatostatin analogue (HR, 0.27 [95% CI, 0.07-0.96]), Lu 177–dotatate plus somatostatin analogue (HR, 0.08 [95% CI, 0.03-0.26], and somatostatin analogues (HR, 0.40 [95% CI, 0.21-0.78]) with higher efficacy than placebo and suggests an overall superiority of combination therapies.
Conclusions and Relevance
The findings from this study suggest that a range of efficient therapies with different safety profiles is available for patients with NETs.
Introduction
The treatment of neuroendocrine tumors (NETs) is an interdisciplinary and dynamic field with many recent innovations from industry and academia. These successful treatments include the mechanistic target of rapamycin inhibitor everolimus,1 the multitargeted receptor tyrosine kinase inhibitor sunitinib,2 the vascular endothelial growth factor antibody bevacizumab,3 the radiolabeled somatostatin analogue lutetium-177 (177Lu)-dotatate,4 and new combinations of previously established therapies.5
Several of these new therapies have demonstrated efficacy in randomized clinical trials (RCTs); however, translation of these results into widespread improved patient care faces several challenges. First, a therapeutic reference standard for treatment of NETs is lacking, and several therapies were compared only with placebo. Second, direct comparison of the most pertinent therapies is incomplete, complicating clinical decision making in selecting one therapy over another. Third, even though innovative and effective combinations of existing therapies have been developed in academic settings, they are often associated with a lack of representation in clinical guidelines.6 We believe this systematic review and network meta-analysis of RCTs will satisfy the need for compiling and evaluating the available evidence on the safety and efficacy of NET therapies.
Methods
The study was designed and conducted according to the Cochrane Handbook for Systematic Reviews of Interventions.7 The report was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement for systematic reviews8,9 and its extension for network meta-analyses.10
Literature Search
We aimed to identify all RCTs comparing therapeutic interventions in NETs. In collaboration with Cochrane Switzerland, we developed a sensitive search algorithm using MeSH terms and text words in combination with an RCT filter (eTable 1 in the Supplement). Using this algorithm, we searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials for studies reported from January 1, 1947, until March 2, 2018. We did not impose language or date restrictions or any exclusion criteria. In addition, we manually searched the trial registries ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform Search Portal (http://apps.who.int/trialsearch) for unpublished eligible trials. We then screened the abstracts from relevant meetings in 2017 and 2018, such as the annual conference of the American Society of Clinical Oncology, the North American Neuroendocrine Tumor Society (NANETS), and the European Neuroendocrine Tumor Society (ENETS), and searched the reference lists of included RCTs and relevant reviews. Key search terms included neuroendocrine tumors, gastrointestinal neoplasms, therapy, and randomized controlled trial.
Study Selection
For the qualitative analysis, we included RCTs comparing a therapeutic intervention with placebo or with an active therapeutic intervention in patients with NETs. For the quantitative analysis, we included all RCTs reporting disease control after 12 months and/or progression-free survival. Eight investigators (R.M.K., M.S., A.K., C.A.S., E.R.C., P.R., M.B., M.A.W.) working in duplicate independently screened titles and abstracts for potentially relevant studies. Five investigators (R.M.K., C.A.S., E.R.C., P.R., M.B.) working in duplicate and then independently screened the full-text report of all potentially relevant studies. Discordances were discussed with a third reviewer (M.A.W.) and resolved by consensus.
Outcomes and Data Extraction
Efficacy outcomes were disease control, progression-free survival, overall survival, and quality of life. Safety outcomes were nonserious and serious adverse events. If values for disease control rate were not available, we used the sum of the rates of complete response, partial response, and stable disease, or 100% minus the rate of disease progression. We extracted absolute values, hazard ratios (HRs), and 95% CIs for progression-free survival and overall survival. We also extracted data on sex, age, tumor type, tumor grading, metastases, functional tumors, follow-up duration, follow-up completeness, sample size calculation, study size, and industry sponsorship. Three investigators (R.M.K., M.S., A.K.) working in duplicate independently extracted all data. Discordances were discussed with a third reviewer (M.A.W.) and resolved by consensus. We contacted the corresponding authors of included RCTs to request additional information if needed, and we assessed the inclusion of RCTs in the recent pertinent guidelines.
Risk of Bias and Quality of Evidence
We assessed the risk of bias for all included RCTs with the Cochrane Risk of Bias Tool7 and evaluated the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).11,12 The details for these assessments are presented in the eMethods in the Supplement.
Statistical Analysis
We performed a network meta-analysis with a frequentist approach using the netmeta package13,14 in R, version 3.5 .15 We analyzed the end points disease control after 12 months and progression-free survival each for pancreatic NET (pNET) and gastrointestinal NET (GI-NET). We applied a continuity correction for studies with a 0 cell count by adding 0.5 to all cell frequencies. We ranked therapies based on P scores, measuring the extent of certainty that a treatment is better than another, averaged over all competing therapies.16
If applicable, we assessed heterogeneity by the between-study-variance τ,2 Cochran Q (weighted sum of squared differences between individual study effects and the pooled effect across studies), and I2 (percentage of variation across studies due to heterogeneity rather than chance). If quantification of heterogeneity was not possible, we fitted fixed-effect models; otherwise, we used random-effects models. We assumed consistency for all networks but could not assess it completely owing to the low number of studies.
We quantified inconsistency by a net split analysis in which direct and indirect estimates were compared and a calculation of the between-design part of Cochran Q analysis. We summarized all results using forest plots with combined effect estimates (ie, odds ratios and HRs, 95% CIs, and size of boxes proportional to the inverse of the SEs).
Two RCTs17,18 in the network meta-analysis did not report HRs. Although the number of events did not match the Kaplan-Meier curves in 1 RCT,17 all reported events could be identified in the Kaplan-Meier curves of the other RCT.18 We contacted the author teams of these trials but did not obtain further data. Thus, we estimated HRs for both RCTs from reconstructed curves by using a Cox proportional hazards regression model and by disregarding the given number of events not matching the Kaplan-Meier curves.17 Two-tailed P values <.05 were considered to indicate statistical significance.
Results
Study Selection
We screened 3671 titles and abstracts and 150 full-text articles and found 38 relevant publications reporting 30 primary RCTs and 8 subgroup analyses (eFigure 1 in the Supplement). One of these primary RCTs19 and 5 of these subgroup analyses,20,21,22,23,24,25 with 1 reported in 2 studies,23,24 were available solely as conference abstracts. A total of 16 RCTs reported disease control and/or progression-free survival and were included in the network meta-analyses. Many of the RCTs were reported in more than 1 publication.
Study Characteristics
The 30 relevant RCTs were conducted in 41 countries on 5 continents and were published between 1980 and 2018. Eleven RCTs included mainly GI-NETs, 9 included mainly pNETs, 8 included GI-NETs and pNETs, and 2 did not specify the type. Overall, 3895 patients were recruited; 22 different therapies were evaluated, including biotherapies, chemotherapies, targeted drugs, locoregional therapies, surgical treatment, and targeted radiopeptide therapy. Most of the 16 RCTs in the network meta-analysis were industry sponsored, and most of the 2944 included patients with metastatic NETs. Further characteristics of included RCTs and patients are provided in eTables 2 and 3 in the Supplement. The characteristics of RCTs and subgroup-analyses not in the network meta-analysis are reported in eTable 4 in the Supplement, and the characteristics of their respective patients are reported in eTable 5 in the Supplement.
Risk of Bias
Among 30 RCTs and 8 subgroup analyses, 20 had low risk for bias in random sequence generation (selection bias, 53%), 20 had low risk for bias in allocation concealment (selection bias, 53%), 21 had low risk for bias in blinding participants and personnel (performance bias, 55%), 19 had low risk for bias in blinding the outcome assessment (detection bias, 50%), 32 had low risk for bias of incomplete outcome data (attrition bias, 84%), and 32 had low risk for bias of selective reporting (reporting bias, 84%) (eTable 6 in the Supplement). Overall, 26 publications (68%) were free of high risk for bias in all of the above-mentioned domains.
Treatment Efficacy in pNETs
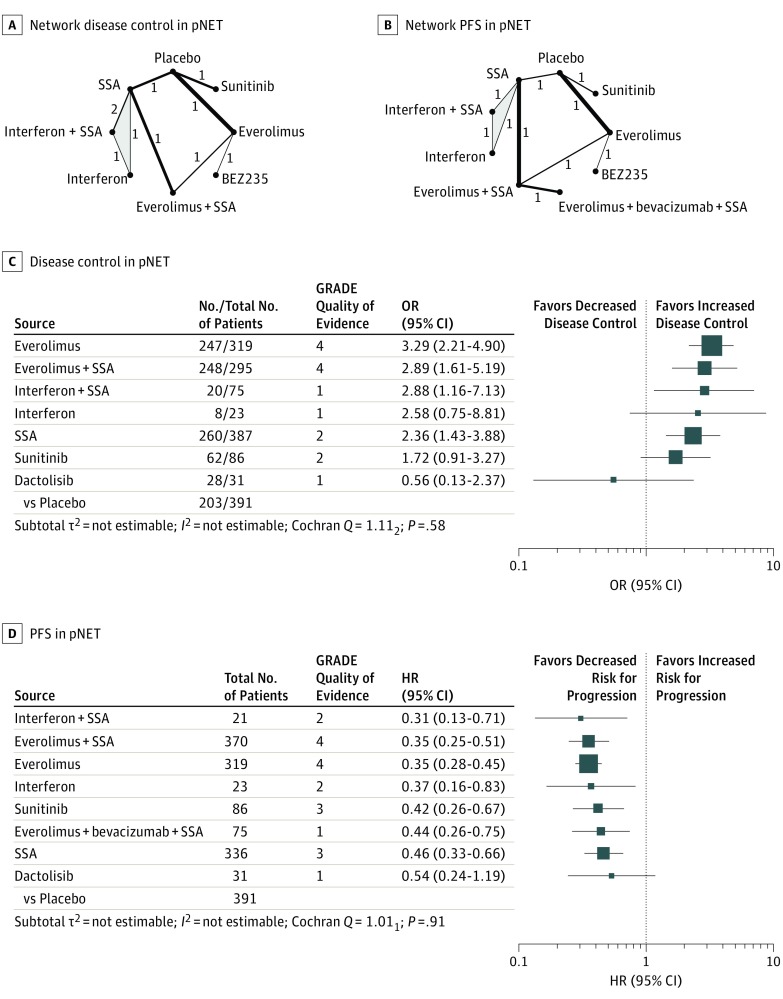
Eight RCTs compared disease control rates for 8 different therapies in pNETs (Figure 1A).2,5,17,26,27,28,29,30,31,32,33 The network meta-analysis found that single therapy with everolimus and combination therapies were highly effective. Specifically, everolimus (P score, 0.82), everolimus plus a somatostatin analogue (P score, 0.73), and interferon plus a somatostatin analogue (P score, 0.71) achieved the highest disease control rates, followed by single treatment with interferon (P score, 0.62), somatostatin analogues (P score, 0.54), sunitinib (P score, 0.39), placebo (P score, 0.13), and dactolisib (P score, 0.06). All therapies except interferon, sunitinib, and dactolisib showed significantly higher disease control rates than placebo (Figure 1C; eFigure 2 in the Supplement).
Figure 1. Treatment Efficacy in Pancreatic Neuroendocrine Tumors (pNETs).
Network plots for disease control (A) and progression-free survival (PFS) (B) in pNETs. The thickness of the edges is proportional to the inverse SEs of the pairwise comparisons, and the numbers indicate the number of studies. One 3-arm study is marked by shading. C and D, Forest plots for disease control and PFS, respectively, in pNETs. The forest plots include only comparisons vs placebo. An odds ratio (OR) larger than 1 indicates increased disease control of the active treatment and a hazard ratio (HR) smaller than 1 indicates a reduced risk for progression for the active treatment. An HR smaller than 1 indicates a reduced risk for progression. All therapies are listed in order of their P scores, with the most effective therapy at the top. Heterogeneity was assessed by the between-study variance τ2 value, Cochran Q with a P value, and I2. Total No. refers to the total number of patients, and No. to the number of patients with disease control. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to rate the quality of evidence of estimates from pairwise and network meta-analysis, where 1 is very low; 2, low; 3, moderate; and 4, high. SSA indicates somatostatin analogue.
In addition, 8 RCTs (one 3-arm trial) assessed progression-free survival for 9 different therapies in pNETs (Figure 1B).2,5,17,26,27,28,29,32,33,34,35 The network meta-analysis found that combination therapies were highly effective, with HRs between 0.31 and 0.35 vs placebo. The lowest hazard for progression was found after treatment with interferon plus a somatostatin analogue (P score, 0.77), followed by everolimus plus a somatostatin analogue (P score, 0.72), everolimus (P score, 0.72), interferon (P score, 0.62), sunitinib (P score, 0.51), everolimus plus bevacizumab plus a somatostatin analogue (P score, 0.44), somatostatin analogues (P score, 0.37), dactolisib (P score, 0.33), and placebo (P score, 0.01). All therapies but dactolisib significantly reduced the hazard for progression compared with placebo (Figure 1D; eFigure 3 in the Supplement). The quality of evidence in pNETs was generally the highest for comparisons including everolimus. The detailed results of the quality assessment are displayed in eTables 7 and 8 in the Supplement.
Treatment Efficacy in GI-NETs
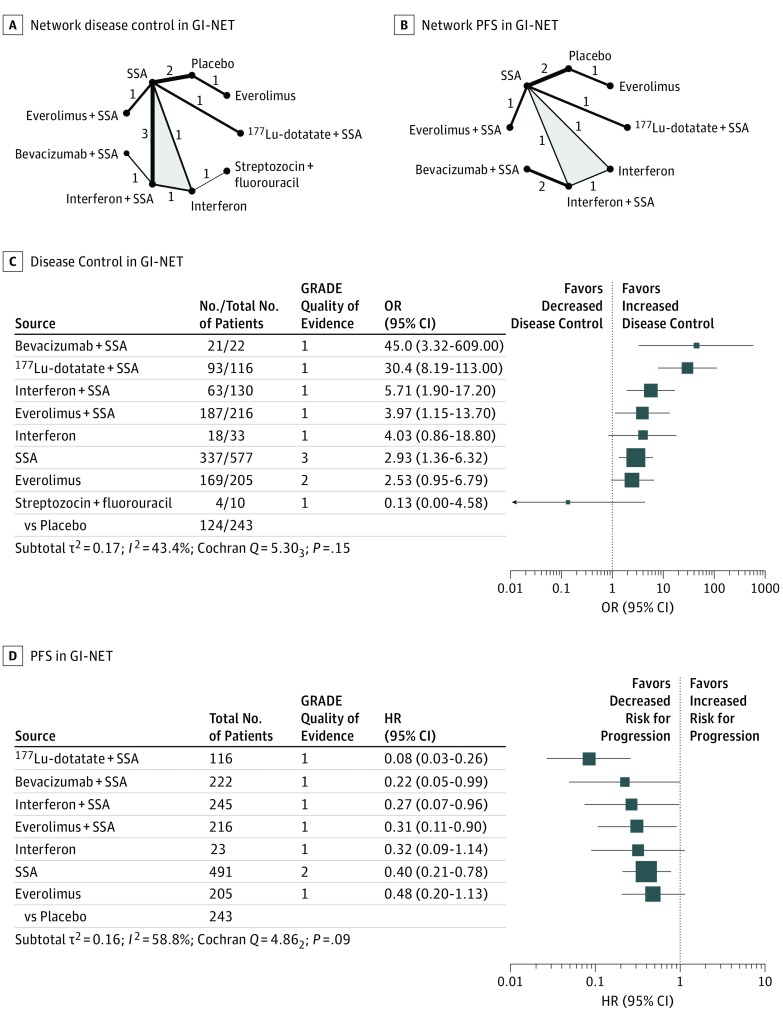
Ten RCTs assessed disease control rates for 9 different therapies in GI-NETs (Figure 2A).1,5,17,18,26,30,31,36,37,38,39,40 Again, the network meta-analysis found that combination therapies were highly effective. Bevacizumab plus a somatostatin analogue resulted in the highest disease control rate (P score, 0.93), followed by 177Lu-dotatate plus a somatostatin analogue (P score, 0.92), interferon plus a somatostatin analogue (P score, 0.66), everolimus plus a somatostatin analogue (P score, 0.53), interferon (P score, 0.52), somatostatin analogues (P score, 0.40), everolimus (P score, 0.39), placebo (P score, 0.12), and streptozocin plus fluorouracil (P score, 0.04). All therapies but interferon, everolimus, and streptozocin plus fluorouracil showed significantly higher disease control rates than placebo (Figure 2C; eFigure 4 in the Supplement).
Figure 2. Treatment Efficacy in Gastrointestinal Neuroendocrine Tumors (GI-NETs).
Network plots for disease control (A) and progression-free survival (PFS) (B) in GI-NETs. The thickness of the edges is proportional to the inverse SEs of the pairwise comparisons, and the numbers indicate the number of studies. One 3-arm study is marked by shading. C and D, Forest plots for disease control and PFS, respectively, in GI-NET. The forest plots include only comparisons vs placebo. An odds ratio (OR) larger than 1 indicates increased disease control of the active treatment and a hazard ratio (HR) smaller than 1 indicates a reduced risk for progression for the active treatment. An HR smaller than 1 indicates a reduced risk for progression. All therapies are listed in order of their P scores, with the most effective therapy at the top. Heterogeneity was assessed by the between-study variance τ2 value, Cochran Q with a P value, and I2. Total No. refers to the total number of patients, and No. to the number of patients with disease control. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to rate the quality of evidence of estimates from pairwise and network meta-analysis, where 1 is very low; 2, low; 3, moderate; and 4, high. 177Lu-dotatate indicates radiolabeled lutetium Lu 177-dotatate; SSA, somatostatin analogue.
Eight RCTs assessed progression-free survival for 8 different therapies in GI-NETs (Figure 2B) and, again, the network meta-analysis found that combination therapies were highly effective, with HRs between 0.08 and 0.31 vs placebo.1,3,5,17,18,26,31,36,37,40 The lowest hazard for progression was found after treatment with 177Lu-dotatate plus a somatostatin analogue (P score, 0.97), followed by bevacizumab plus a somatostatin analogue (P score, 0.68), interferon plus a somatostatin analogue (P score, 0.59), everolimus plus a somatostatin analogue (P score, 0.53), interferon (P score, 0.50), somatostatin analogues (P score, 0.38), everolimus (P score, 0.33), and placebo (P score, 0.02). All therapies but interferon and everolimus significantly reduced the hazard for progression compared with placebo (Figure 2D; eFigure 5 in the Supplement). The quality of evidence in GI-NETs was generally the highest for comparisons including somatostatin analogues. The detailed results of the quality assessment are displayed in eTables 9 and 10 in the Supplement.
Disease Control, Progression-Free Survival, and Overall Survival
Twelve RCTs reported data on disease control and progression-free survival,1,2,5,17,18,26,27,28,29,36,37,41 and both outcomes were generally positively associated (eFigure 6 in the Supplement). Moreover, 11 RCTs reported data on overall survival (eTable 11 in the Supplement),2,19,29,30,32,33,34,35,42,43,44 and 8 RCTs reported both progression-free survival and overall survival.1,2,3,29,34,36,37,42 In each of these RCTs, superiority of a therapy regarding progression-free survival was associated with superiority regarding overall survival.
Quality of Life and Safety
Ten RCTs reported effects on quality of life,1,2,26,30,31,33,35,36,40,42,44,45,46,47,48 and 7 of these quantified changes for 7 different therapies with the Quality of Life Questionnaire C30 of the European Organization for Research and Treatment of Cancer.2,26,30,31,33,35,36,42,44,45,46 Of these, telotristat etiprate had the greatest effect on improving quality of life, followed by somatostatin analogues (eTable 12 in the Supplement). Furthermore, 12 RCTs and 7 subgroup analyses1,26,27,28,29,31,32,37,39,40,41,42,45,46,49,50 reported frequencies of adverse events for 12 different therapies, of which dactolisib showed the highest and interferon plus a somatostatin analogue showed the lowest rates of serious adverse events (83.9% vs 3.0%) (Table). Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (grade 1, mild; grade 2, moderate; grade 3, severe or medically significant; grade 4, life-threatening).51
Table. Percentage of Patients With Adverse Events According to Treatment.
| Treatment | Grades, No./Total No. (%)a | Source, Reference No. | |
|---|---|---|---|
| 3-4 | All | ||
| Dactolisib | 26/31 (83.9) | 31/31 (100) | 28 |
| Everolimus + somatostatin analogue | 67/98 (68.4) | 81/98 (82.7) | 27, 41 |
| Capecitabine + streptozocin + cisplatin | 25/40 (62.5) | 37/40 (92.5) | 42 |
| Everolimus | 309/521 (59.3) | 291/316 (92.1) | 1, 27, 29, 31, 32, 40 |
| Capecitabine + streptozocin | 18/43 (41.9) | 41/43 (95.3) | 42 |
| 177Lu-dotatate + somatostatin analogue | 46/111 (41.4) | 105/111 (94.6) | 37 |
| Hepatic arterial chemoembolization | 3/12 (25.0) | 11/12 (91.7) | 49 |
| Somatostatin analogue | 72/344 (20.9) | 226/324 (69.8) | 26, 37, 39, 41, 45, 50 |
| Placebo | 92/480 (19.2) | 278/363 (76.6) | 26, 29, 32, 45, 46, 50 |
| Hepatic arterial embolization | 2/14 (14.3) | 12/14 (85.7) | 49 |
| Interferon + somatostatin analogue | 1/33 (3.0) | 7/33 (21.2) | 39 |
| Telotristat | 0 | 79/90 (87.8) | 46 |
Abbreviation: 177Lu-dotatate, radiolabeled lutetium Lu 177–dotatate.
Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events: grade 1, mild; grade 2, moderate; grade 3, severe or medically significant; and grade 4, life-threatening.
Representation in International Guidelines
Only 14 of the existing 30 RCTs and 8 subgroup analyses (37%) on NET treatment were included in both the latest NANETS and ENETS consensus guidelines (eTables 2 and 4 in the Supplement); thus, 63% were not included in both guidelines. Specifically, 12 of 27 publications (44%) with industry sponsoring1,2,3,5,17,26,29,30,31,32,33,35,36,37,40,46,49 and 2 of 11 publications (18%) without industry sponsoring43,48 were included in both guidelines (eTables 2 and 4 in the Supplement).
Discussion
This systematic review identified 30 RCTs that randomized 3895 patients to 22 different therapies. To our knowledge, this represents the most comprehensive overview of the available safety and efficacy data for NET therapies, and its main findings can be summarized as follows.
First, the results suggest a superiority of combination therapies, especially of those including somatostatin analogues. In pNETs, somatostatin analogues plus interferon, everolimus, or everolimus plus bevacizumab were highly efficacious. The certainty of evidence for these therapies was variable and was the highest for somatostatin analogues plus everolimus. In GI-NETs, somatostatin analogues plus 177Lu-dotatate, bevacizumab, interferon, or everolimus were highly efficacious. Also, the certainty of evidence for these therapies was variable and was highest for somatostatin analogues plus 177Lu-dotatate.
Second, the results suggest a range of monotherapies that are superior to placebo, including everolimus, interferon, and sunitinib in pNETs, and somatostatin analogues in pNETs and GI-NETs. Conversely, the results did not demonstrate efficacy superior to that of placebo for dactolisib in pNETs or for interferon or everolimus in GI-NETs. The highest quality of evidence was available for everolimus in pNETs, alone or in combination with somatostatin analogues.
Third, the results indicate that NET therapies have a broad range of risk for adverse events and effects on quality of life. Because systemic treatment is commonly noncurative for NETs, adverse events and quality of life are priorities. The results of this study may help to put the available safety data for NET therapies into perspective. The findings may guide treatment choice, initiate preventive measures, and result in increased patient surveillance. In addition, they demonstrate the need for more research in assessing adverse events and effects on quality of life for NET therapies.
Strengths and Limitations
This study has limitations. We conducted a comprehensive literature search with a sensitive search algorithm and an extensive manual search of reference lists and conference proceedings. We therefore consider it unlikely that we missed relevant RCTs. However, we could not obtain additional unpublished data and are aware that a substantial amount of information is not available to the public. Thus, we cannot rule out publication bias.
When using the available information for therapeutic decisions in treatment of NETs, we propose to consider the following points regarding indirectness, transitivity, risk of bias, inconsistency, incoherence, and imprecision. First, meta-analyses are based on the assumption of directness, in which populations, therapies, and outcomes of included studies are aligned with population, therapies, and outcomes targeted by the meta-analysis. Our meta-analysis targeted all available therapies and included only studies reporting disease control and/or progression-free survival. Both factors ensured a certain degree of directness. Yet, indirectness was introduced by RCTs including mixed populations of patients with pNETs and GI-NETs. We highlight all comparisons that were affected by indirectness (eTables 4-7 in the Supplement) to allow incorporation of this fact into clinical decision making.
Second, network meta-analyses are also based on the assumption of transitivity, in which the included studies are similar enough to build a network. In this study, the well-defined populations and outcomes resulted in a network with high overall transitivity. Yet, the different types of interferons3,17,18,30,38,39 and somatostatin analogues3,5,17,18,26,27,30,31,36,37,39 introduced intransitivity for the loop of comparisons of interferon, somatostatin analogues, and their combination, but had no association with the certainty of evidence for the rest of the network.
Third, some RCTs had a high risk of bias due to absent blinding, including the RCTs evaluating the 3 most efficacious therapies in GI-NETs: somatostatin analogues plus bevacizumab,3,18 plus 177Lu-dotatate,37 or plus interferon.17 Absent blinding has been shown to be associated with an average exaggeration of estimated therapeutic effects of approximately 9%.52 However, the therapeutic effect for the 3 aforementioned therapies compared with placebo substantially exceeds 9% and they most likely represent the superior therapies in GI-NETs, although the extent of superiority needs to be interpreted with caution.
Fourth, consistency describes the agreement between estimates of different studies for a specific comparison, while coherence describes agreement between direct and indirect estimates for a specific comparison. Owing to the relatively low number of RCTs, the assessment of incoherence and inconsistency was limited. We identified 6 comparisons in which indirect and direct estimates differed considerably, without being statistically significant, and 2 cases of inconsistency. Two RCTs compared somatostatin analogues with placebo,26,36 and 3 RCTs compared somatostatin analogues with somatostatin analogues plus interferon.17,30,39 Likely owing to different types of somatostatin analogues and interferons, the RCTs found different effects regarding disease control and progression-free survival.
Fifth, the low number of RCTs compared with the number of interventions induced imprecision to several comparisons, manifesting as wide 95% CIs that include or are close to a null effect. A statistically significant effect does not automatically represent a clinically relevant effect, and the consequence of imprecision is that wide 95% CIs might include significant but clinically irrelevant effects. As clinical relevance often depends on an individual patient's situation, we highlighted all comparisons that were affected by imprecision (eTables 4-7 in the Supplement) to allow incorporation of this fact into clinical decision making.
We used the GRADE system to assess the confidence in effect estimates for all comparisons, depending on indirectness, transitivity, risk of bias, inconsistency, incoherence, and imprecision. We incorporated the certainty of evidence in the main results of our analysis (eTables 4-7 in the Supplement) to highlight the most robust findings for further use in clinical judgment.
Sixth, we used the end points disease control and progression-free survival for all network analyses, instead of overall survival. Although overall survival is arguably the most relevant clinical end point, it is used less frequently because it requires a larger number of patients and longer follow-up. Use of overall survival also prevents crossover trial designs and might be confounded by the effect of salvage therapies used after disease progression.53 In NETs, progression-free survival has been shown to be well correlated with overall survival,54 and the RCTs included in the present study revealed the same correlation. Using disease control and progression-free survival instead of overall survival in this study allowed including more therapies into the network meta-analyses, which we believe represents the preferred approach.
Implications
Our results have implications for clinicians, guideline committees, and researchers. First, clinical decisions should be based on the best available evidence. The present results provide a comprehensive overview of the existing evidence on NET therapies as well as the best possible comparison of therapies that have not been directly compared in RCTs. Using this approach, the certainty of evidence is incorporated into the results to assist in decision making. Safety and efficacy results should both be incorporated into the treatment decision, while in addition the safety results may aid in the decision to establish preventive measures and increase the surveillance for known toxic effects.
Furthermore, the results support the adaption of clinical guidelines. Although there is no requirement to incorporate all evidence from RCTs into clinical guidelines, this systematic review presents an overview of the existing evidence from which guideline makers can choose. For example, evidence from RCTs on alosetron,55 dactolisib,28 capecitabine,42 temozolomide,19 and surgical ligation devices56 in NETs has not been integrated into many guidelines, and combination therapies could be better represented. Everolimus plus a somatostatin analogue showed high efficacy in pNETs and is widely recommended only by the Carcinoid-Neuroendocrine Tumor Society Canada.57 It is recommended solely for functionally active pNETs by the ENETS, but not by the NANETS.58,59 Interferon plus a somatostatin analogue showed high efficacy and low toxic effects in pNETs and is recommended for pNETs with reservation by the ENETS, but not by NANETS.58,59 Conversely, 177Lu-dotatate plus a somatostatin analogue showed high efficacy in GI-NETs and is recommended by the NANETS, but not by the ENETS.58,60 Bevacizumab plus a somatostatin analogue showed high efficacy in GI-NET, but is not recommended by either the NANETS or ENETS.58,60
Similarly, NET guidelines by the European Society for Medical Oncology,61 National Comprehensive Cancer Network,62 Scottish Neuroendocrine Tumour Group,63 and UK and Ireland Neuroendocrine Tumour Society64 do not recommend combinations of everolimus, interferon, bevacizumab, or 177Lu-dotatate plus a somatostatin analogue. Yet, the Scottish Neuroendocrine Tumour Group guidelines mention possible benefits of bevacizumab or everolimus plus a somatostatin analogue in pNET.63
The present results may guide future research by highlighting necessary head-to-head comparisons and facilitating their trial design.65 Specifically, dactolisib and everolimus plus bevacizumab plus a somatostatin analogue have only been compared with 1 other active therapy in pNET yet, while everolimus, everolimus plus a somatostatin analogue, bevacizumab plus a somatostatin analogue, 177Lu-dotatate plus a somatostatin analogue, and streptozocin plus fluorouracil have only been compared with 1 other active therapy in GI-NETs. Sunitinib and everolimus have been compared only with placebo in pNETs and GI-NETs respectively, and, to our knowledge, head-to-head comparisons with active therapies in RCTs have not yet been performed. When designing such head-to-head comparisons, the estimated associations from our network meta-analysis can help to select the reference therapy and approximate the required patient numbers. Particularly, because the present results identified 7 therapies in pNETs and 5 therapies in GI-NETs with higher efficacy than placebo, comparisons with placebo as a reference are discouraged for the future. Because of their proven efficacy and central role in current comparisons, somatostatin analogues represent the logical reference compound for further RCTs. Moreover, the quality assessment of currently available RCTs revealed that further studies should incorporate blinding to avoid overestimation of effects and improve the overall quality of evidence in the field.
Conclusions
Herein, we present a systematic review and network meta-analysis of available RCTs evaluating the safety and efficacy of therapies for NETs. This overview of what we believe to be the most pertinent and current evidence demonstrates a range of efficient therapies with different safety profiles that are available for patients with NETs and may facilitate informed clinical decision making, drafting of guidelines, and planning of future research.
eMethods. Detailed Methods
eFigure 1. Flowchart of Search Results
eFigure 2. Disease Control in pNET
eFigure 3. PFS in pNET
eFigure 4. Disease Control in GI-NET
eFigure 5. PFS in GI-NET
eFigure 6. Ranking of Treatment Efficacies for Disease Control and Progression-Free Survival
eTable 1. Search Strategies
eTable 2. Characteristics of Randomized Controlled Trials Included in the Network Meta-analysis
eTable 3. Participants’ Characteristics of Randomized Controlled Trials Included in the Network Meta-analysis
eTable 4. Characteristics of Randomized Controlled Trials Not Included in the Network Meta-analysis
eTable 5. Participants’ Characteristics of Randomized Controlled Trials Not Included in the Network Meta-analysis
eTable 6. Risk of Bias Summary: Authors' Judgments About Each Risk of bias item for Each Included Study
eTable 7. Estimates of Effects and Quality Ratings for Disease Control in Pancreatic Neuroendocrine Tumors (pNET)
eTable 8. Estimates of Effects and Quality Ratings for Progression-Free Survival in pNET
eTable 9. Estimates of Effects and Quality Ratings for Disease Control in Gastrointestinal Neuroendocrine Tumors (GI-NET)
eTable 10. Estimates of Effects and Quality Ratings for Progression-Free Survival in GI-NET
eTable 11. Overall Survival in Months According to the Treatment
eTable 12. Changes in Quality of Life During Treatment Based on EORTC QLQ-30
References
- 1.Yao JC, Fazio N, Singh S, et al. ; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group . Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968-977. doi: 10.1016/S0140-6736(15)00817-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raymond E, Dahan L, Raoul JL, et al. . Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501-513. doi: 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Guthrie KA, Moran C, et al. . Phase III prospective randomized comparison trial of depot octreotide plus interferon alfa-2b versus depot octreotide plus bevacizumab in patients with advanced carcinoid tumors: SWOG S0518. J Clin Oncol. 2017;35(15):1695-1703. doi: 10.1200/JCO.2016.70.4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strosberg JR, Wolin EM, Chasen B, et al. . NETTER-1 phase III: Progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177-Lu-dotatate. J Clin Oncol. 2016;34(4)(suppl):194-194. doi: 10.1200/jco.2016.34.4_suppl.19426503197 [DOI] [Google Scholar]
- 5.Pavel ME, Hainsworth JD, Baudin E, et al. ; RADIANT-2 Study Group . Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005-2012. doi: 10.1016/S0140-6736(11)61742-X [DOI] [PubMed] [Google Scholar]
- 6.Boudoulas KD, Leier CV, Geleris P, Boudoulas H. The shortcomings of clinical practice guidelines. Cardiology. 2015;130(3):187-200. doi: 10.1159/000371572 [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; http://handbook.cochrane.org. Updated September 2018. Accessed January 8, 2019. [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94. doi: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 10.Hutton B, Salanti G, Caldwell DM, et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 11.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 12.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 13.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R (Use R!). Geneva, Switzerland: Springer; 2015. doi: 10.1007/978-3-319-21416-0 [DOI] [Google Scholar]
- 14.Rücker G, Schwarzer G, Krahn U, König J netmeta: Network Meta-Analysis using Frequentist Methods. 2017; R-Package version 0.9-7. https://cran.r-project.org/web/packages/netmeta/index.html. Accessed January 1, 2018.
- 15.Al-Qahtani KH, Tunio MA, Asiri MA, et al. . Comparative clinicopathological and outcome analysis of differentiated thyroid cancer in Saudi patients aged below 60 years and above 60 years. Clin Interv Aging. 2016;11:1169-1174. doi: 10.2147/CIA.S107881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faiss S, Pape UF, Böhmig M, et al. ; International Lanreotide and Interferon Alfa Study Group . Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21(14):2689-2696. doi: 10.1200/JCO.2003.12.142 [DOI] [PubMed] [Google Scholar]
- 18.Yao JC, Phan A, Hoff PM, et al. . Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26(8):1316-1323. doi: 10.1200/JCO.2007.13.6374 [DOI] [PubMed] [Google Scholar]
- 19.Kunz PL, Catalano PJ, Nimeiri H, et al. . A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol. 2018;36(15)(suppl):4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan AT, Caplin ME, Pavel ME, et al. . Effects of lanreotide autogel/depot (LAN) in pancreatic neuroendocrine tumors (pNETs): a subgroup analysis from the CLARINET study. J Clin Oncol. 2015;33(3)(suppl):233. [Google Scholar]
- 21.Phan AT, Caplin ME, Pavel ME, et al. . Effects of lanreotide autogel/depot (LAN) in patients with neuroendocrine tumors (NETs) age 65 or younger versus older than age 65: subgroup analyses from the CLARINET study. J Clin Oncol. 2015;33(3)(suppl):36.25349295 [Google Scholar]
- 22.Dasari A, Phan AT, Caplin ME, et al. . Lanreotide depot/autogel (LAN) in midgut neuroendocrine tumors (NETs): a subgroup analysis from the CLARINET study. J Clin Oncol. 2015;33(15)(suppl):4104. doi: 10.1200/jco.2015.33.15_suppl.e15177 [DOI] [Google Scholar]
- 23.Fisher GA, Wolin EM, Kunz P, et al. . Safety and efficacy of lanreotide depot versus placebo in neuroendocrine tumor patients with a history of carcinoid syndrome and prior octreotide therapy. Am J Gastroenterol. 2015;110(suppl 1):1007. [Google Scholar]
- 24.Fisher GA, Wolin EM, Kunz P, et al. . Efficacy and safety of lanreotide depot vs placebo in patients with neuroendocrine tumor and a history of carcinoid syndrome and prior octreotide therapy. Pancreas. 2016;45(3):475. [Google Scholar]
- 25.Anselmo L, Shaheen M, Casellini C, et al. . Safety and efficacy of lanreotide depot vs placebo in neuroendocrine tumor patients with a history of carcinoid syndrome and prior octreotide therapy. J Oncol Pharm Pract. 2016;22(2)(suppl 1):19-20. [Google Scholar]
- 26.Caplin ME, Pavel M, Ćwikła JB, et al. ; CLARINET Investigators . Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224-233. doi: 10.1056/NEJMoa1316158 [DOI] [PubMed] [Google Scholar]
- 27.Kulke MH, Ruszniewski P, Van Cutsem E, et al. . A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol. 2017;28(6):1309-1315. doi: 10.1093/annonc/mdx078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar R, Garcia-Carbonero R, Libutti SK, et al. . Phase II study of BEZ235 versus everolimus in patients with mammalian target of rapamycin inhibitor-naive advanced pancreatic neuroendocrine tumors. Oncologist. 2018;23(7):766-e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao JC, Shah MH, Ito T, et al. ; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group . Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514-523. doi: 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold R, Rinke A, Klose KJ, et al. . Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3(8):761-771. doi: 10.1016/S1542-3565(05)00481-7 [DOI] [PubMed] [Google Scholar]
- 31.Phan AT, Dasari A, Liyanage N, Cox D, Pitman Lowenthal S, Wolin EM Tumor response in the CLARINET study of lanreotide depot vs placebo in patients with metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J Clin Oncol. 2016;34(4 suppl):434. doi: 10.1200/jco.2016.34.4_suppl.434 [DOI] [Google Scholar]
- 32.Yao JC, Pavel M, Lombard-Bohas C, et al. . Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 Study. J Clin Oncol. 2016;34(32):3906-3913. doi: 10.1200/JCO.2016.68.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinik A, Bottomley A, Korytowsky B, et al. . Patient-reported outcomes and quality of life with sunitinib versus placebo for pancreatic neuroendocrine tumors: results from an international phase III trial. Target Oncol. 2016;11(6):815-824. doi: 10.1007/s11523-016-0462-5 [DOI] [PubMed] [Google Scholar]
- 34.Kulke MH, Niedzwiecki D, Foster NR, et al. . Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (alliance). Pancreas. 2016;45(3):477. [Google Scholar]
- 35.Faivre S, Niccoli P, Castellano D, et al. . Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol. 2017;28(2):339-343. [DOI] [PubMed] [Google Scholar]
- 36.Rinke A, Müller HH, Schade-Brittinger C, et al. ; PROMID Study Group . Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656-4663. doi: 10.1200/JCO.2009.22.8510 [DOI] [PubMed] [Google Scholar]
- 37.Strosberg J, El-Haddad G, Wolin E, et al. ; NETTER-1 Trial Investigators . Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125-135. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberg K, Norheim I, Alm G. Treatment of malignant carcinoid tumors: a randomized controlled study of streptozocin plus 5-FU and human leukocyte interferon. Eur J Cancer Clin Oncol. 1989;25(10):1475-1479. doi: 10.1016/0277-5379(89)90107-7 [DOI] [PubMed] [Google Scholar]
- 39.Kölby L, Persson G, Franzén S, Ahrén B. Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg. 2003;90(6):687-693. doi: 10.1002/bjs.4149 [DOI] [PubMed] [Google Scholar]
- 40.Pavel ME, Singh S, Strosberg JR, et al. . Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(10):1411-1422. doi: 10.1016/S1470-2045(17)30471-0 [DOI] [PubMed] [Google Scholar]
- 41.Castellano D, Bajetta E, Panneerselvam A, et al. ; RADIANT-2 Study Group . Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study. Oncologist. 2013;18(1):46-53. doi: 10.1634/theoncologist.2012-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer T, Qian W, Caplin ME, et al. . Capecitabine and streptozocin ± cisplatin in advanced gastroenteropancreatic neuroendocrine tumours. Eur J Cancer. 2014;50(5):902-911. doi: 10.1016/j.ejca.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 43.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326(8):519-523. doi: 10.1056/NEJM199202203260804 [DOI] [PubMed] [Google Scholar]
- 44.Meyer T, Qian W, Valle JW, et al. . Capecitabine and streptozocin ± cisplatin for gastroenteropancreatic neuroendocrine tumours: predictors of long-term survival in the NET01 trial. Ann Oncol. 2016;27(suppl 6):vi136-vi148. doi: 10.1093/annonc/mdw369.31 [DOI] [Google Scholar]
- 45.Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA; ELECT Study Group . Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): a randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22(9):1068-1080. doi: 10.4158/EP151172.OR [DOI] [PubMed] [Google Scholar]
- 46.Kulke MH, Hörsch D, Caplin ME, et al. . Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35(1):14-23. doi: 10.1200/JCO.2016.69.2780 [DOI] [PubMed] [Google Scholar]
- 47.Jacobsen MB, Hanssen LE. Clinical effects of octreotide compared to placebo in patients with gastrointestinal neuroendocrine tumours: report on a double-blind, randomized trial. J Intern Med. 1995;237(3):269-275. doi: 10.1111/j.1365-2796.1995.tb01175.x [DOI] [PubMed] [Google Scholar]
- 48.O’Toole D, Ducreux M, Bommelaer G, et al. . Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer. 2000;88(4):770-776. doi: [DOI] [PubMed] [Google Scholar]
- 49.Maire F, Lombard-Bohas C, O’Toole D, et al. . Hepatic arterial embolization versus chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: a prospective randomized study. Neuroendocrinology. 2012;96(4):294-300. doi: 10.1159/000336941 [DOI] [PubMed] [Google Scholar]
- 50.Oberg K, Norheim I, Theodorsson E, Ahlman H, Lundqvist G, Wide L. The effects of octreotide on basal and stimulated hormone levels in patients with carcinoid syndrome. J Clin Endocrinol Metab. 1989;68(4):796-800. doi: 10.1210/jcem-68-4-796 [DOI] [PubMed] [Google Scholar]
- 51.US Dept of health and human services: Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0, 2017: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed January 8, 2019.
- 52.Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol. 2007;36(4):847-857. doi: 10.1093/ije/dym087 [DOI] [PubMed] [Google Scholar]
- 53.Saad ED, Buyse M. Statistical controversies in clinical research: end points other than overall survival are vital for regulatory approval of anticancer agents. Ann Oncol. 2016;27(3):373-378. doi: 10.1093/annonc/mdv562 [DOI] [PubMed] [Google Scholar]
- 54.Imaoka H, Sasaki M, Takahashi H, et al. . Progression-free survival as a surrogate endpoint in advanced neuroendocrine neoplasms. Endocr Relat Cancer. 2017;24(9):475-483. doi: 10.1530/ERC-17-0197 [DOI] [PubMed] [Google Scholar]
- 55.Saslow SB, Scolapio JS, Camilleri M, et al. . Medium-term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut. 1998;42(5):628-634. doi: 10.1136/gut.42.5.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakata H, Iwakiri R, Ootani A, et al. . A pilot randomized control study to evaluate endoscopic resection using a ligation device for rectal carcinoid tumors. World J Gastroenterol. 2006;12(25):4026-4028. doi: 10.3748/wjg.v12.i25.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh S, Asa SL, Dey C, et al. . Diagnosis and management of gastrointestinal neuroendocrine tumors: an evidence-based Canadian consensus. Cancer Treat Rev. 2016;47:32-45. doi: 10.1016/j.ctrv.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 58.Pavel M, O’Toole D, Costa F, et al. ; Vienna Consensus Conference participants . ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103(2):172-185. doi: 10.1159/000443167 [DOI] [PubMed] [Google Scholar]
- 59.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. ; North American Neuroendocrine Tumor Society . Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557-577. doi: 10.1097/MPA.0b013e31828e34a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. . The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46(6):707-714. doi: 10.1097/MPA.0000000000000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Öberg K, Knigge U, Kwekkeboom D, Perren A; ESMO Guidelines Working Group . Neuroendocrine gastro-entero-pancreatic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii124-vii130. [DOI] [PubMed] [Google Scholar]
- 62.Shah MH, Kulke MH, Goldner WS, et al. Neuroendocrine tumors, version 3.2018. NCCN Clinical Practice Guidelines in Oncology 2018; https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed October 06, 2018, 2018.
- 63.Bouvier C, Bradshaw N, Chong P, et al. Consensus guidelines for the management of patients with neuroendocrine tumors (v.1.1). http://www.woscan.scot.nhs.uk/wp-content/uploads/FINAL-PUBLISHED-SCONET-Guideline-v1.1-July-2015.pdf. Updated July 2015. Accessed February 15, 2018, 2018.
- 64.Ramage JK, Ahmed A, Ardill J, et al. ; UK and Ireland Neuroendocrine Tumour Society . Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61(1):6-32. doi: 10.1136/gutjnl-2011-300831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salanti G, Nikolakopoulou A, Sutton AJ, et al. . Planning a future randomized clinical trial based on a network of relevant past trials. Trials. 2018;19(1):365. doi: 10.1186/s13063-018-2740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methods
eFigure 1. Flowchart of Search Results
eFigure 2. Disease Control in pNET
eFigure 3. PFS in pNET
eFigure 4. Disease Control in GI-NET
eFigure 5. PFS in GI-NET
eFigure 6. Ranking of Treatment Efficacies for Disease Control and Progression-Free Survival
eTable 1. Search Strategies
eTable 2. Characteristics of Randomized Controlled Trials Included in the Network Meta-analysis
eTable 3. Participants’ Characteristics of Randomized Controlled Trials Included in the Network Meta-analysis
eTable 4. Characteristics of Randomized Controlled Trials Not Included in the Network Meta-analysis
eTable 5. Participants’ Characteristics of Randomized Controlled Trials Not Included in the Network Meta-analysis
eTable 6. Risk of Bias Summary: Authors' Judgments About Each Risk of bias item for Each Included Study
eTable 7. Estimates of Effects and Quality Ratings for Disease Control in Pancreatic Neuroendocrine Tumors (pNET)
eTable 8. Estimates of Effects and Quality Ratings for Progression-Free Survival in pNET
eTable 9. Estimates of Effects and Quality Ratings for Disease Control in Gastrointestinal Neuroendocrine Tumors (GI-NET)
eTable 10. Estimates of Effects and Quality Ratings for Progression-Free Survival in GI-NET
eTable 11. Overall Survival in Months According to the Treatment
eTable 12. Changes in Quality of Life During Treatment Based on EORTC QLQ-30