Evidence shows that subjective toxicities are underreported in patients with cancer and that collection of this information directly from patients can improve the reliability and precision of symptomatic adverse events detection. This study reported the feasibility of using the complete set of subjective symptoms from PRO‐CTCAE library developed by the National Cancer Institute in patients on phase I clinical trials.
Abstract
The patient‐reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE) complements capture of symptomatic adverse events (AEs) by clinicians. Previous trials have typically used a limited subset of relevant symptomatic AEs to reduce patient burden. We aimed to determine the feasibility of administering all 80 AEs included in the PRO‐CTCAE library by approaching consecutive patients enrolled in a large academic phase I program at three points in time. Here, we report a preplanned analysis after enrolling the first 20 patients. All items were answered on 51 of 56 potential visits (adherence 91%). Three (5%) additional PRO‐CTCAE assessments were partially completed, and two (4%) were missed because of conflicting appointments. No patient withdrew consent or chose not to complete the assessments once enrolled on study. Future trials of experimental drugs that incorporate the PRO‐CTCAE should consider using this unselected approach to identify adverse events more completely.
Introduction
The standard for reporting adverse events (AEs) in cancer clinical trials is the Common Terminology Criteria for Adverse Events (CTCAE) [1]. A growing body of evidence indicates subjective toxicities are underreported in patients with cancer and that collection of this information directly from patients can improve the reliability and precision of symptomatic AE detection [2], [3], [4], [5], [6]. The National Cancer Institute has developed a library of 78 patient‐reported symptomatic AEs to collect subjective symptoms directly from patients, called the patient‐reported outcomes version of the CTCAE (PRO‐CTCAE) [7]. For each AE there are between one and three items to assess the frequency, severity, and/or interference with activities related to that AE, for a total of 124 items [7]. Most studies that have incorporated the PRO‐CTCAE have included fewer than 20 AEs that were chosen to pair with the clinician‐graded AEs, which are prospectively monitored in the clinical trial protocol [4], [5], [8], [9]. Although using selected symptomatic AEs reduces patient burden, it could also lead to incomplete reporting, especially of unanticipated AEs. No previous study used the complete PRO‐CTCAE item library.
Methods
We initiated a study to assess patient‐reported outcomes in a phase I clinical trial setting using the complete PRO‐CTCAE item library, with an interim feasibility analysis after enrolling the first 20 patients. Research ethics committee approval was obtained. All consecutive English‐speaking patients enrolled in solid tumor phase I trials at the Princess Margaret Cancer Centre, Toronto, Canada, a specialized academic cancer center, were approached by a research assistant not involved with phase I trials, who tracked the patient's schedule and identified appropriate outpatient visits for PRO‐CTCAE completion. All 78 PRO‐CTCAE symptomatic AEs were assessed at three time points: baseline (prior to the initiation of investigational therapy), midcycle 1, and midcycle 2. Patients provided informed consent and completed the PRO‐CTCAE using tablet computers, a method previously found equivalent to paper surveys [10], [11]. A specific web‐based application with branching logic to minimize the number of questions administered to patients was developed for this study. The patients’ responses to the PRO‐CTCAE were not made known to the clinical team. The application was designed to ensure all items were answered by mandating the response to each item within a group be provided before the patient could move on to the next group of items. If needed, the application could be paused and resumed later during the same visit. The duration of time required to complete the questionnaire was measured but not captured because of a technical software issue. The planned total sample size for this ongoing study is 200 patients. Here, we report the preplanned interim feasibility analysis of this approach.
Results
Twenty patients were enrolled between May 12 and June 9, 2017. A total of 25 patients were approached for this study; four patients declined (acceptance of 20/24, 83%), and one patient was missed because of a scheduling conflict. One patient declined to participate because he was already involved in a clinical trial, another because of fatigue; two patients provided no specific reasons. Patient characteristics are shown in Table 1.
Table 1. Patient characteristics.
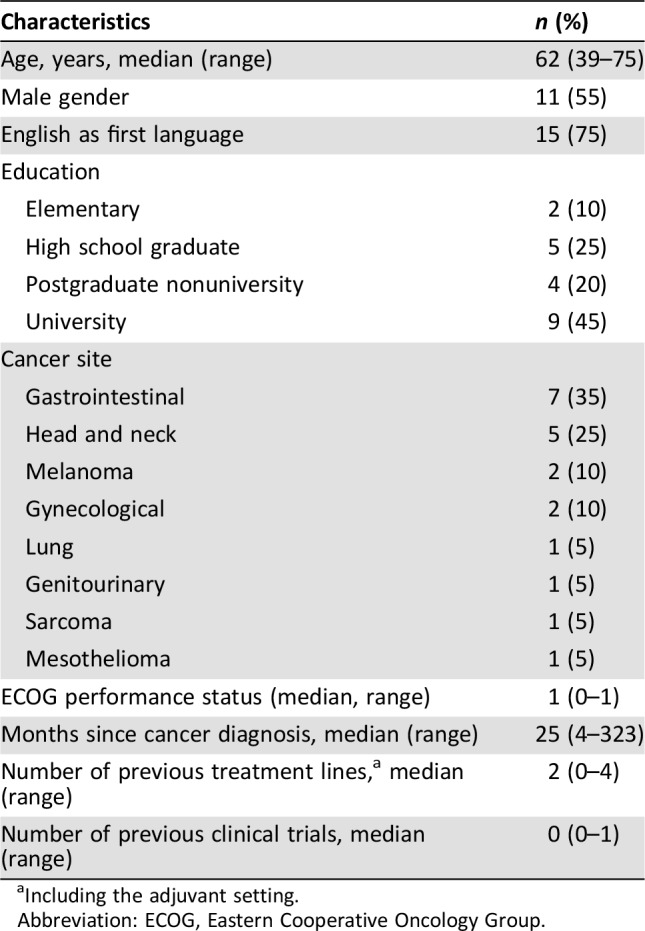
Including the adjuvant setting.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
The completion rate by patient is provided in Table 2. Twelve patients completed all items on all three visits. Four patients who completed all items for the first two visits were taken off study for disease progression prior to the third visit. Three patients had an incomplete assessment at one visit, and two patients missed their scheduled PRO‐CTCAE assessments because of scheduling conflicts (one patient who missed a visit also had one incomplete assessment). Overall, from 56 planned PRO‐CTCAE assessments, 51 (91%) were fully completed, 3 (5%) were partially completed, and 2 (4%) were missed. Although no formal patient interviews were performed after completion of the survey, informal patient and study personnel feedback did not identify the application as burdensome or report other issues.
Table 2. Patient adherence.
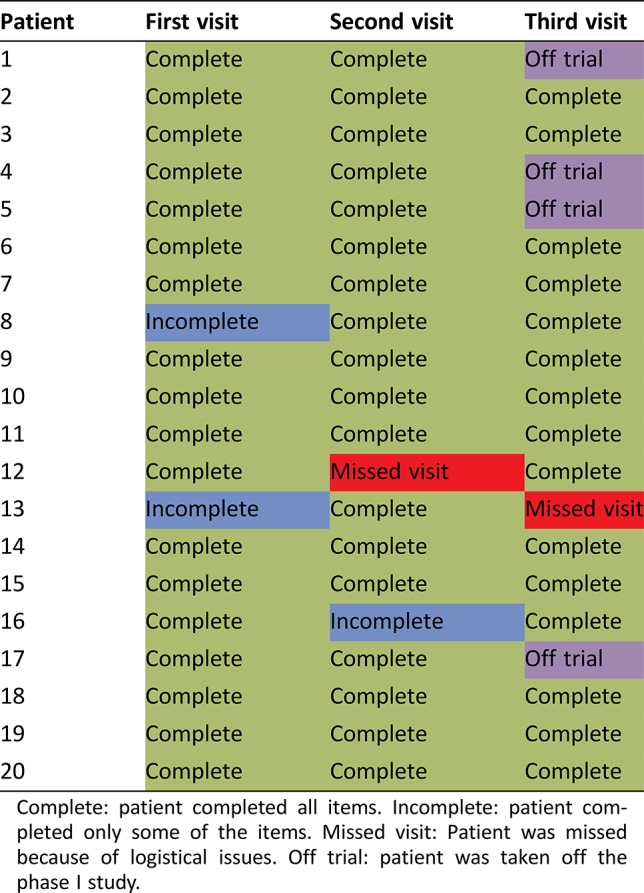
Complete: patient completed all items. Incomplete: patient completed only some of the items. Missed visit: Patient was missed because of logistical issues. Off trial: patient was taken off the phase I study.
Discussion
Our study has demonstrated that phase I trial patients are able to complete the full PRO‐CTCAE at multiple time points. Patient and clinician perspectives of adverse symptoms that occur in clinical trials can provide complementary information that together may better define the toxicity profile [12]. Patients may view tolerability differently than clinicians [3]. Integration of PROs into clinical care can improve patients’ quality of life, reduce emergency department admissions, and lead to improved survival [13], [14]. International regulatory agencies acknowledge the value of incorporating the patient experience into regulatory decision‐making [15].
The PRO‐CTCAE is considered a promising tool to assess patient‐reported symptomatic AEs [16]. Considerable heterogeneity exists in the selection of PRO‐CTCAE AEs administered in previous studies [4], [5], [6], [7], [8]. The choice of symptomatic AEs for active monitoring in clinical trials is based upon pretrial information, mechanism of action, and AEs seen in similar classes of agents. However, by the very nature of clinical trials, in which experimental agents are being evaluated, unexpected adverse events can occur that are not predicted by previous data. Thus, subtle treatment effects may be missed.
Use of the entire PRO‐CTCAE item library has been previously avoided in large cancer trials because of concerns regarding patient burden and low adherence. Our data, though preliminary, show that patients are willing and able to complete the full item library at three time points. Previous studies showed comparable adherence rates on consecutive visits regardless of the number of symptomatic AEs assessed, which supports our findings [4], [5], [6], [8], [9]. Capturing all patient‐reported outcomes provides a more complete description of the patient experience and provides a systematic approach to identify treatment‐emergent symptomatic events.
The main limitation of this study is the small, highly selected patient population. Participants in phase I programs have better performance status and fewer comorbidities and are highly motivated to participate in clinical trials. Data on survey completion times were not recorded, and formal debriefing interviews were not conducted, which would have confirmed initial feasibility. The adherence of “real‐world” patients with cancer to reporting the complete PRO‐CTCAE item library is unknown and should be assessed. Adherence to PRO‐CTCAE assessments may have been enhanced by the presence of a dedicated research assistant and a user‐friendly tablet application. Adherence for more than three visits is unknown.
Conclusion
The adherence of patients with cancer in a phase I clinical trials setting to reporting the complete PRO‐CTCAE item library on consecutive visits was high and comparable to previous reports of selected symptomatic AEs. Future phase I trials of experimental therapies or agents with a high risk of unexpected side effects that incorporate the PRO‐CTCAE should consider using this unselected approach.
Disclosures
The authors indicated no financial relationships.
References
- 1.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) . Rockville, MD: National Cancer Institute. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Updated March 1, 2018. Accessed June 7, 2018.
- 2.Di Maio M, Galoo C, Leighl NB et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol 2015;33:910–915. [DOI] [PubMed] [Google Scholar]
- 3.Henon C, Lissa D, Paoletti X et al. Patient‐reported tolerability of adverse events in phase 1 trials. ESMO Open 2017;2:e000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basch E, Iasonos A, McDonough T et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire‐based study. Lancet Oncol 2016;7:903–909. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Wood WA, Schrag D et al. Feasibility and clinical impact of sharing patient‐reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer treatment trial. Clin Trials 2016;13:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dueck AC, Mendoza TR, Mitchell SA et al. Validity and reliability of the US National Cancer Institute's Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). JAMA Oncol 2015;1:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Patient‐Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE) . Rockville, MD: National Cancer Institute. https://healthcaredelivery.cancer.gov/pro‐ctcae/. Updated 2018. Accessed June 7, 2018.
- 8.Basch E, Pugh SL, Dueck AC et al. Feasibility of patient reporting of symptomatic adverse events via the Patient‐Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE) in a chemoradiotherapy cooperative group multicenter clinical trial. Int J Radiat Oncol Biol Phys 2017;98:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basch E, Dueck AC, Rogak LJ et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 2017;3:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett AV, Dueck AC, Mitchell SA et al. Mode equivalence and acceptability of tablet computer, interactive voice response system, and paper‐based administration of the U.S. National Cancer Institute's Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). Health Qual Life Outcomes 2016;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Use of Electronic Informed Consent in Clinical Investigations: Questions and Answers . Guidance for Institutional Review Boards, Investigators, and Sponsors. Silver Spring, MD: U.S. Food and Drug Administration; 2015. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm436811.pdf. Accessed June 7, 2018.
- 12.Basch E, Jia X, Heller G et al. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Natl Cancer Inst 2009;101:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basch E, Deal AM, Dueck AC et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluetz PG, O'Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol 2018;19:e267–e274. [DOI] [PubMed] [Google Scholar]
- 16.Kluetz PG, Chingos DT, Basch EM et al. Patient‐reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute's Patient‐Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). Am Soc Clin Oncol Educ Book 2016;35:67–73. [DOI] [PubMed] [Google Scholar]


