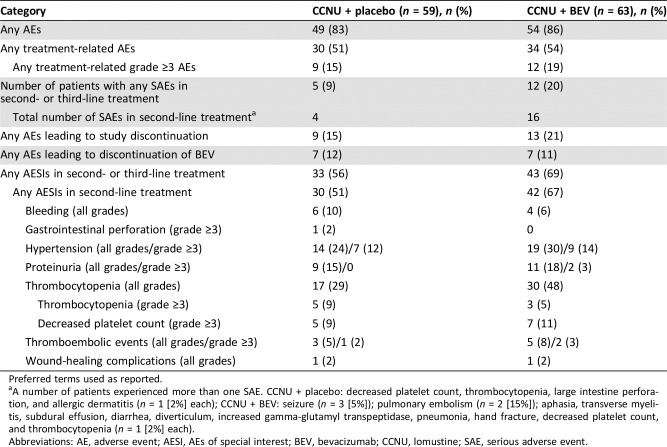
Table 2. Overview of safety in second‐ and third‐line treatments (safety population).
Preferred terms used as reported.
A number of patients experienced more than one SAE. CCNU + placebo: decreased platelet count, thrombocytopenia, large intestine perforation, and allergic dermatitis (n = 1 [2%] each); CCNU + BEV: seizure (n = 3 [5%]); pulmonary embolism (n = 2 [15%]); aphasia, transverse myelitis, subdural effusion, diarrhea, diverticulum, increased gamma‐glutamyl transpeptidase, pneumonia, hand fracture, decreased platelet count, and thrombocytopenia (n = 1 [2%] each).
Abbreviations: AE, adverse event; AESI, AEs of special interest; BEV, bevacizumab; CCNU, lomustine; SAE, serious adverse event.