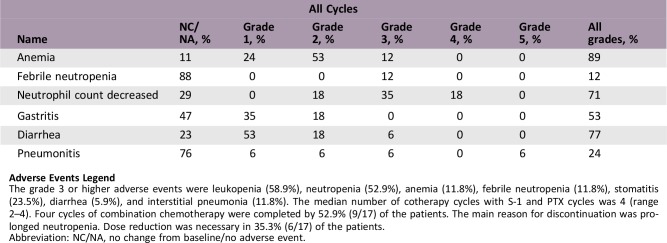
Adverse Events Legend
The grade 3 or higher adverse events were leukopenia (58.9%), neutropenia (52.9%), anemia (11.8%), febrile neutropenia (11.8%), stomatitis (23.5%), diarrhea (5.9%), and interstitial pneumonia (11.8%). The median number of cotherapy cycles with S‐1 and PTX cycles was 4 (range 2–4). Four cycles of combination chemotherapy were completed by 52.9% (9/17) of the patients. The main reason for discontinuation was prolonged neutropenia. Dose reduction was necessary in 35.3% (6/17) of the patients.
Abbreviation: NC/NA, no change from baseline/no adverse event.