Figure 1.
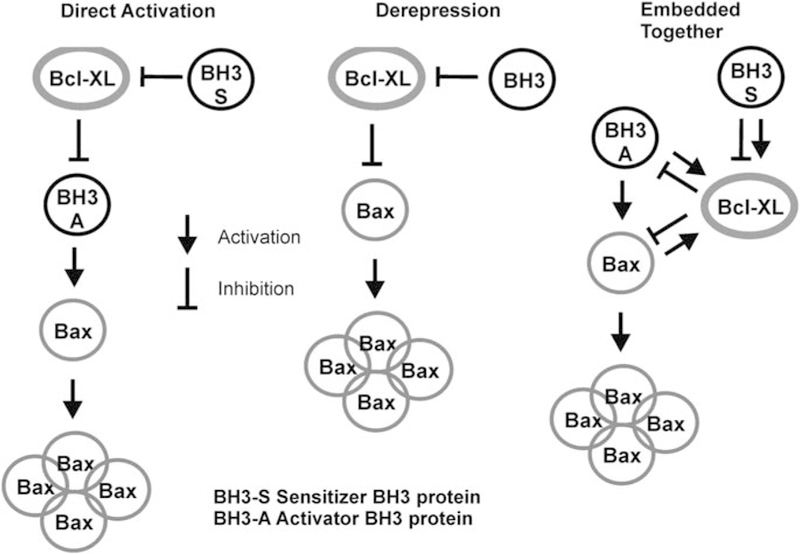
Models of regulation of apoptosis by Bcl-2 family proteins. Simplified versions of the three models of apoptosis regulation to emphasize the different roles postulated for interactions between the three different classes of Bcl-2 proteins (BH3-only proteins, antiapoptotic members such as Bcl-XL and membrane-permeabilizing proteins such as Bax). These diagrams neglect the role of the membrane in permitting a comparison of the direct activation and derepression models (no role for membrane) with the protein interactions of the embedded together model. The direct activation and derepression models postulate different functional states for Bax or Bak; in the former, these proteins are inactive and must be activated by BH3-only proteins, whereas in the latter they are constitutively active and must be repressed by antiapoptotic proteins such as Bcl-XL. Thus, they propose different mechanisms of action for Bcl-XL inhibition of apoptosis. The embedded together model recognizes both of these interactions. In the derepression model, BH3-only proteins are distinguished by their differential affinity to the varying antiapoptotic Bcl-2 family members, but in the direct activation model they are divided into two classes: those that directly activate Bax or Bak (activators) and those that prevent Bcl-XL from binding to activators (sensitizers). The embedded together model also recognizes these two different classes, although it considers the fact that both classes have a role in ‘activating’ Bcl-XL by causing its membrane insertion. The consequence of the subsequent interaction in the membrane differs: BH3-only activator proteins are sequestered by Bcl-XL and therefore cannot activate Bax or Bak, whereas BH3-only sensitizer proteins prevent Bcl-XL from binding to activator proteins, thereby inhibiting Bcl-XL.
