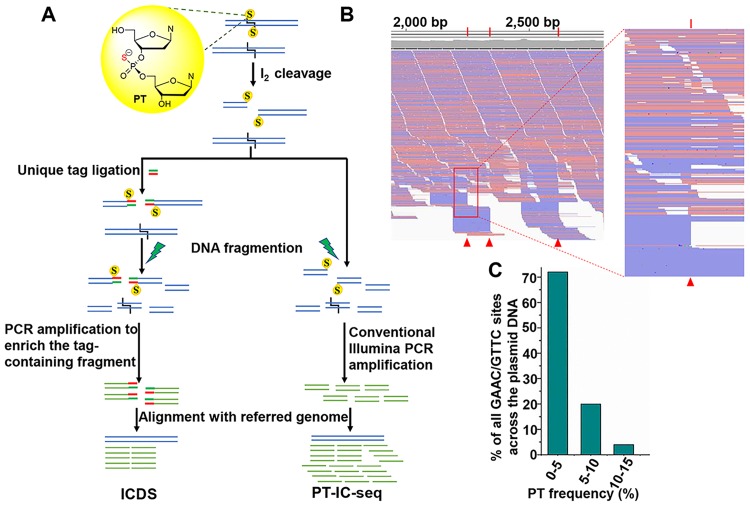
Fig 2. PT-IC-seq, a new method for quantitatively PT mapping.
(A) Flowchart of ICDS and PT-IC-seq. The treatment of bistranded PT modified DNA with I2 will produce a double-strand break. In the ICDS method, after treatment with I2, the resulted double-strand break is then processed and ligated to a unique double-stranded oligodeoxynucleotide tag, followed sonicated to an optimal range (150–350 bp) in length. After Illumina adaptors addition, the DNA is subjected to PCR amplification to enrich the I2 cleaved fragments. And finally, subjected to Illumina sequencing. While, in the PT-IC-seq method, after treatment with I2, the resulted double-strand broke into long DNA fragments were directly fragmented. Following addition of Illumina adaptors, the DNA is subsequently subjected to PCR amplification and sequencing. (B) The snapshot of genome browser (IGV) representing a partially PT modified sites in pBlueScript SK(+) plasmid extract from S. enterica serovar Cerro 87. The top track shows counts of 5’ ends in the selected region. Blue segments represent reads mapped to the minus strand and red segments represent reads mapped to the plus strand. The red triangle marks GpsAAC/GpsTTC site. (C) Statistics analysis of the PT modification frequency in pBlueScript SK(+) plasmid extract from S. enterica serovar Cerro 87. All of the PT modified sites in the pBlueScript SK(+) plasmid extract from S. enterica serovar Cerro 87 are partially-PT modified, and more than 70% of the PT modified sites with the PT ratio lower than 5%.