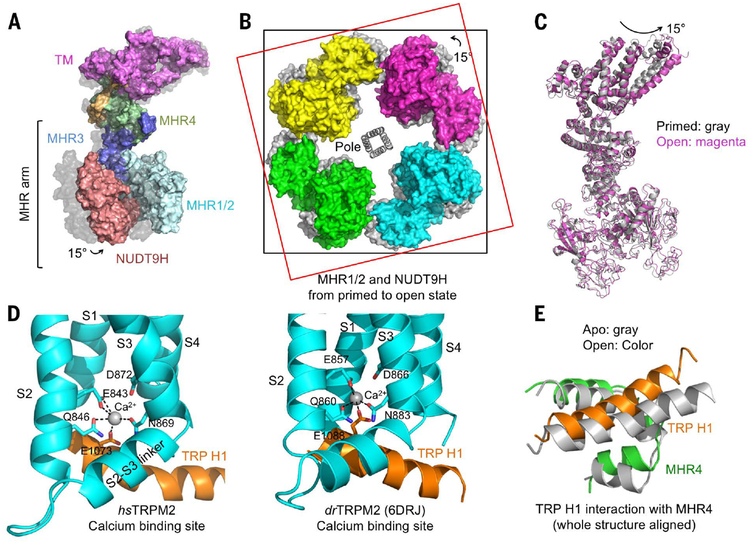
Fig. 4. Global conformational changes of hsTRPM2 during its opening.
(A) Side view of one subunit in surface representation, showing a 15° rotation from the primed state (gray surface) to the open state (colored surface). The entire MHR arm moves. (B) Top view of overlaid tetramers in the open state (colored surface) and the primed state (gray surface). Two squares help to indicate the rotation. (C) Superimposed TRPM2 structures in the primed (gray) and open (magenta) states. (D) Coordination of Ca2+ by residues in TRP H1, S2, and S3 in both hsTRPM2 and drTRPM2 (PDB 6DRJ) (28). (E) TRP H1 and MHR4 are positioned in close proximity via direct interactions, shown as a global superposition between the apo (gray) and open (colored) states. TRP H1 tilts as a consequence of Ca2+ binding, establishing a molecular coupling between TRP H1 and the cytosolic domain.