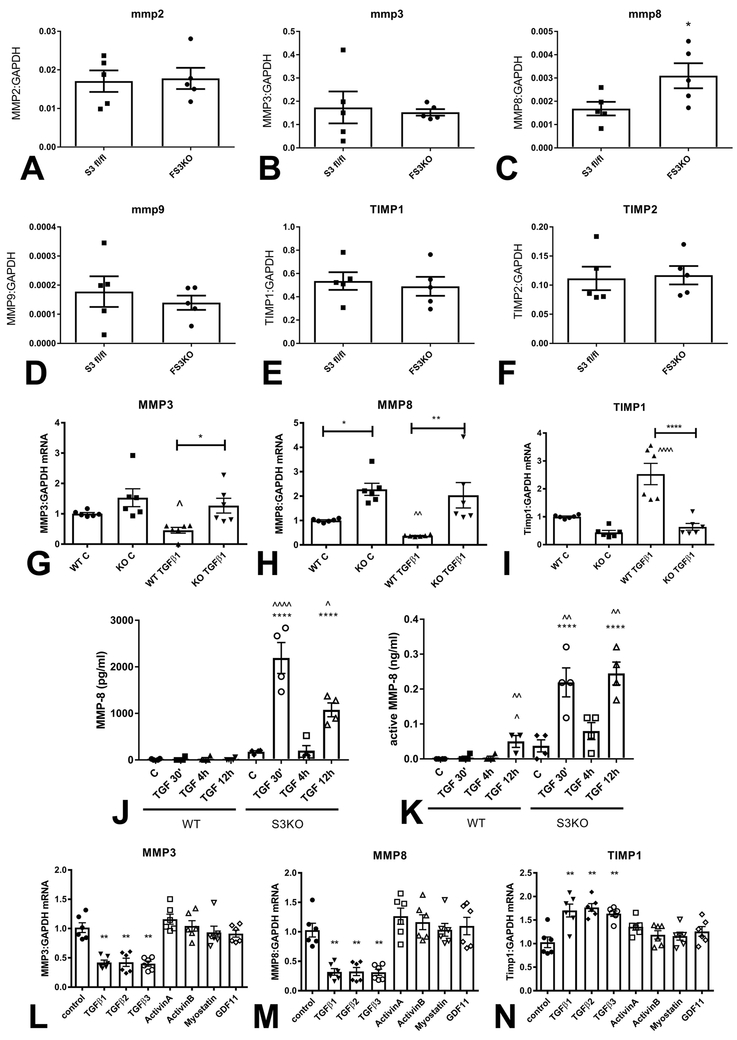
Figure 6: Smad3 signaling mediates the matrix-preserving effects of TGF-β in cardiac fibroblasts.
Fibroblasts harvested from FS3KO and Smad3 fl/fl hearts after 7 days of TAC had comparable MMP2 (A) and MMP3 (B) mRNA expression levels, but exhibited markedly elevated MMP8 expression (C, *p<0.05, n=5/group). MMP9 (D), TIMP1 (E) and TIMP2 (F) expression levels were comparable between Smad3 fl/fl and FS3KO fibroblasts. G-I: Effects of TGF-β on MMP and TIMP expression in cardiac fibroblasts cultured in collagen pads are dependent on Smad3. In WT cells, TGF-β1 stimulation suppressed synthesis of MMP3 (G) and MMP8 (H) and induced TIMP-1 (I) expression (**p<0.01 vs. control, n=6/group). In contrast, TGF-β1 had no significant effects on MMP3, MMP8 and TIMP1 expression in Smad3 KO fibroblasts (^p<0.05, ^^p<0.01 vs. corresponding control). J-K: WT cardiac fibroblasts had low levels of MMP8 protein and activity in the supernatant, in the presence or absence of TGF-β. In contrast, Smad3 KO cells exhibited marked release of MMP8 protein and activity 30 min and 12h after TGF-β1 stimulation (****p<0.0001 vs. corresponding WT cells; ^p<0.05, ^^p<0.01, ^^^^p<0.0001 vs. unstimulated cells, n=4/group). The findings suggested that Smad3 signaling restrains release of MMP8 protein and activity by cardiac fibroblasts. L-N: Effects of Smad3 activating members of the TGF-β superfamily on expression of MMP3, MMP8 and TIMP1. Only TGF-β1, β2 and β3, and not activin-A, activin-B, myostatin and GDF-11 suppressed MMP3 (L) and MMP8 (M) expression, and induced TIMP-1 synthesis (N) in fibroblasts cultured in collagen pads (**p<0.01 vs. control, n=5/group).