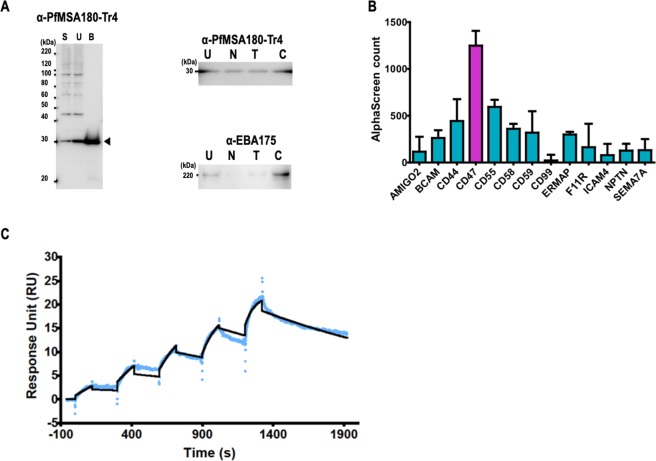
Figure 4.
PfMSA180 interacts with CD47, a human erythrocyte surface protein. (A) Erythrocyte binding activity of native PfMSA180 protein compared to EBA175. Left panel: S, parasite culture supernatant; U, erythrocyte unbound fraction; B, untreated erythrocyte bound fraction. Arrowhead indicates the bound fragment of PfMSA180. Right panels: U, unbound fraction to the untreated erythrocytes; N, neuraminidase-treated erythrocytes; T, trypsin-treated erythrocytes; C, chymotrypsin-treated erythrocytes. Original data is included in the Supplementary Data File 1. (B) AlphaScreen reactivity profile of recombinant PfMSA180-Tr4 to 13 GST-fused recombinant proteins. Biotinylated recombinant PfMSA180-Tr4 was mixed with each of 13 GST-fused erythrocyte surface proteins and incubated for 1 h at 26 °C to form a protein-protein complex. Subsequently, a suspension of streptavidin-coated donor-beads and anti-GST acceptor-beads mixture in reaction buffer was added. The mixture was incubated for 12 h at 26 °C in dark. This allowed the donor- and acceptor-beads to optimally bind to biotin and GST, respectively. Upon illumination of this complex, a luminescence signal at 620 nm was detected by the EnVision plate reader (PerkinElmer) and the result was expressed as AlphaScreen counts. HisGST was used to set the background and was subtracted from each sample signal. Each bar represents the average AlphaScreen counts in quintuplicate with error bars representing SE of the mean. CD47 had the highest mean signal. (C) Sensorgram of SPR single-cycle kinetic analysis. Recombinant CD47 was immobilized on a CM5 chip and used as the ligand while recombinant PfMSA180-Tr4 was used as analyte. The blue curve represents the data-generated sensorgram while the black curve indicates line of fit used to calculate kinetics parameters. All assays were performed at an increasing protein concentration of 0.125, 0.25, 0.5, 1, and 2 µM at 120 s contact time and 180 s dissociation time. The dissociation time in the last cycle was extended to 580 s.