Figure 2.
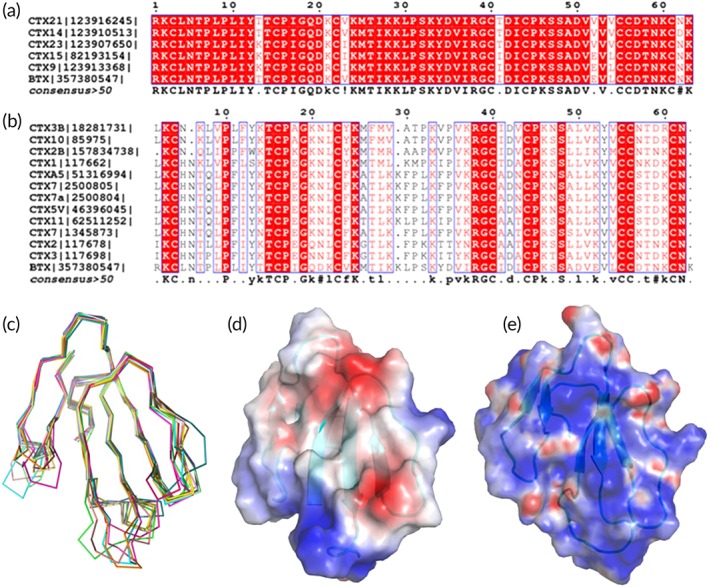
Comparison of β‐Cardiotoxin with other three‐finger toxins (3FTXs). (a) Sequence alignment of β‐cardiotoxin with all closely related 3FTX homologs (>90% identity). (b) Sequence alignment of β‐cardiotoxin with the conventional CTXs (about 50% identities). The alignment is done by ClustalW and figures are prepared by ESPRIPT. (c) Comparison of β‐cardiotoxin with its structural homologs using DALI search. β‐Cardiotoxin [3PLC] (chocolate), Hemachatoxin [3VTS] (60) (orange), cardiotoxin V [1KXI] (61) (red), cytotoxin 4 [4OM5] (62) (dark green), cytotoxin 3 [2BHI] (63) (light green), muscarinic m1‐toxin1 [2VLW] (64) (dark brown), cytotoxin 3 [1XT3] (65) (blue), cardiotoxin‐A3 [1H0J] (66) (light blue), bucain [2H8U] (67) (light brown), cytotoxin 2 [4OM4] (62) (yellow), muscarinic m1‐toxin1 [3NEQ] (68) (magenta), muscarinic toxin MT1 [4DO8] (68) (cyan), and ringhalexin (18) [4ZGY] (green). (d) Electrostatic surface potential of β‐cardiotoxin and Cardiotoxin V (61) (e). The electrostatic surface potentials in this paper are calculated using Pymol plug‐in APBS and scaled at ±10 kT. β‐Cardiotoxin does not have the cationic/hydrophobic central β‐sheet feature, which is critical for CTX's cytolytic activity.
