Figure 3.
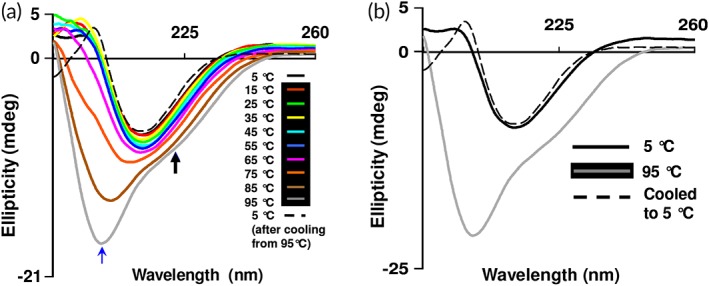
Thermal (un)folding studies of β‐cardiotoxin. (a) Effect of temperature on the secondary structure of β‐cardiotoxin. The protein was dissolved in MilliQ water (0.5 mg/mL) and far‐UV CD spectra were recorded using a 0.1 cm path‐length cuvette 5°C (after cooling from 95°C, black dotted line). The blue and black arrows indicate the new bands arising at 203 and 222 nm. (b) Refolding of β‐cardiotoxin after thermal denaturation.
