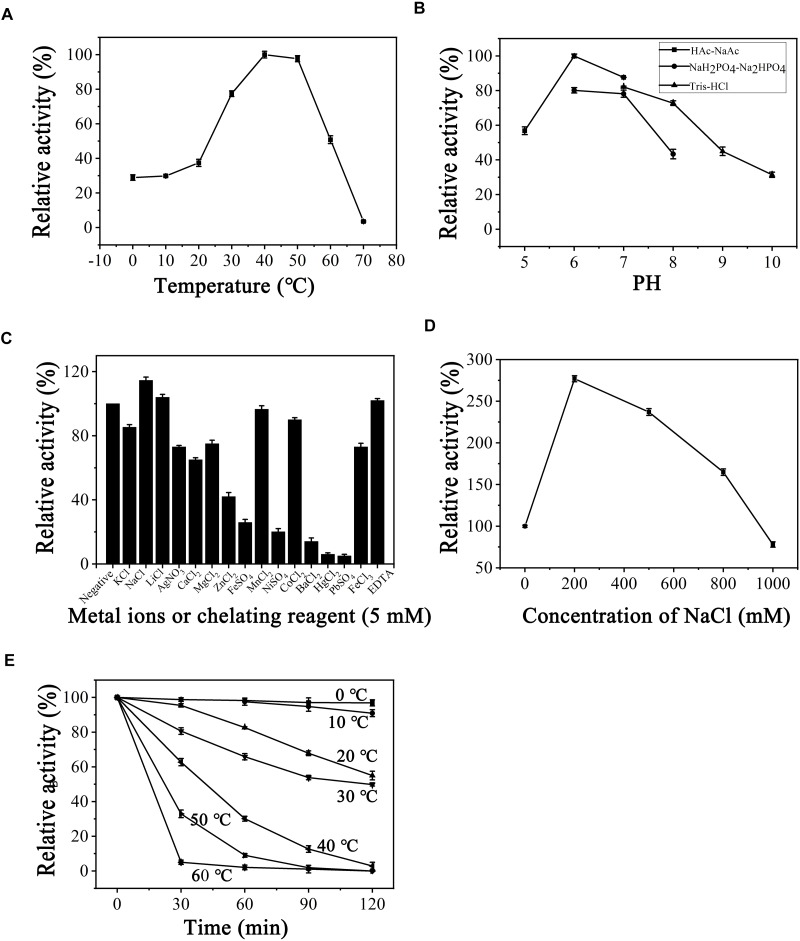
FIGURE 4.
Biochemical conditions for recombinant PB2SF. (A) Effect of temperature. The enzyme activity of PB2SF was measured using the ΔD disaccharide as a substrate in 50 mM NaH2PO4-Na2HPO4 buffer (pH 8.0), at different temperatures for 10 min. The data are shown as percentages of the activity obtained at 40°C (100%). (B) Effect of pH. The activity of PB2SF against ΔD was measured in buffers with pH values from 5 to 10 at 40°C for 10 min. The data are shown as percentages of the activity obtained in the HAc-NaAc buffer at pH 6.0 (100%). (C) Effects of metal ions. The activities of PB2SF against ΔD were measured in the HAc-NaAc buffer (pH 6.0) containing 5 mM of various metal ions at 40°C for 10 min. Data are shown as a percentage of the activity obtained in the buffer without the tested metal ions. (D) Effects of NaCl concentrations. The activity of PB2SF against ΔD was measured in HAc-NaAc buffer (pH 6.0) containing 0–1 M NaCl at 40°C for 10 min. Data are shown as a percentage of the activity obtained in the buffer without NaCl. (E) Thermostability of PB2SF. The enzyme in 50 mM HAc-NaAc buffer (pH 6.0) was preincubated for 0–120 min at temperatures from 0 to 60°C, and the residual activity against ΔD was estimated at 40°C. Data are shown as the activity relative to that of untreated PB2SF. Error bars represent the means of triplicate analyses ± SD.