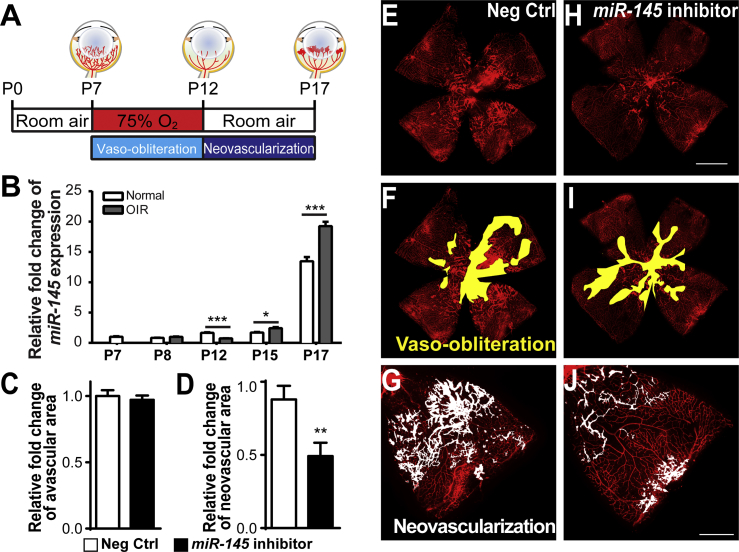
Figure 1.
miR-145 Suppresses Pathological Neovascularization in Mouse Retinas with Oxygen-Induced Retinopathy
(A) Schematic diagram of the oxygen-induced retinopathy (OIR) model. Neonatal mice were exposed to 75% of oxygen from postnatal day 7 (P7) to 12. Vessel loss was induced during oxygen exposure, and maximal neovascularization was induced at P17, 5 days after the mice were returned to room air. (B) Expression levels of miR-145 were analyzed in OIR retinas compared with age-matched room air control retinas at different time points. MiR-145 expression was significantly reduced in OIR retinas at P12, yet was upregulated at both P15 and P17 compared with normoxic retinas, normalized to U6 small nuclear RNA (snRNA) as the control (n = 6 per group). (C–J) Intravitreal injection of the miR-145 inhibitor or the non-targeting negative control was performed in OIR mouse eyes at P12, with the miR-145 inhibitor injected into one eye and the negative control in the contralateral eye, followed by analysis of the retinal vasculature at P17. Quantitative analysis of vaso-obliteration (C) and pathological neovascularization (D) showed that miR-145 inhibitor treatment significantly reduced neovascularization in OIR retinas compared with negative control treatment of contralateral retinas, with no significant difference in vaso-obliteration (n = 16 per group). Representative retinal whole mounts were stained with Griffonia simplicifolia isolectin B4 (IB4) from P17 OIR mice with injection of negative control (E) or miR-145 inhibitor (H); areas of vaso-obliteration (F, negative controls, and I, miR-145 inhibitor) and neovascular tufts (G, negative controls, and J, miR-145 inhibitor) are labeled in yellow and white, respectively. Scale bars represent 1 mm (B) and 500 μm (F). Neg Ctrl, negative control RNA oligomers. Data are presented as means ± SEM. *p ≤ 0.05; **p < 0.005; ***p ≤ 0.001.