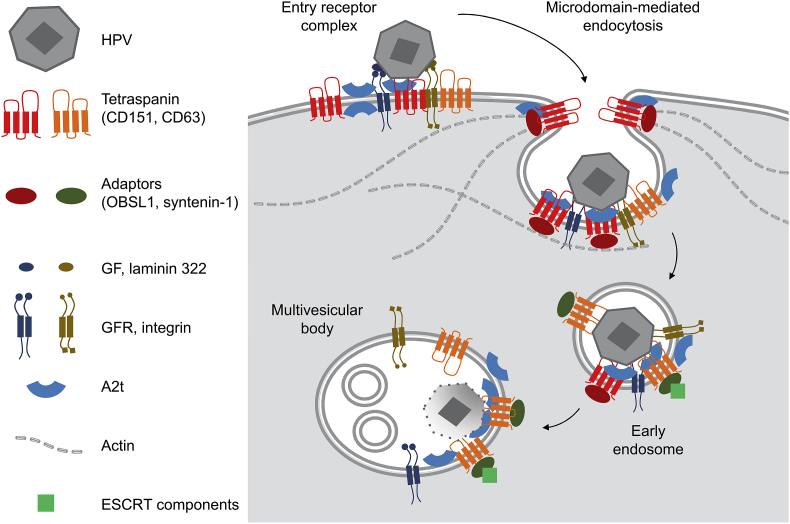
Fig. 1.
Schematic diagram of HPV16 endocytosis and trafficking to multivesicular bodies. After priming and activation of signaling cascades, the HPV16 capsid interacts with the entry receptor complex composed of growth factor receptors, laminin-binding integrins, tetraspanins and annexins. Upon HPV binding to this complex, a non-canonical and clathrin independent endocytic process is initiated, which involves actin, the cytoskeletal adaptor protein OBSL1, the annexin A2 heterotetramer and tetraspanin CD151. Internalized viruses are then routed to acidified multivesicular bodies. This early trafficking again involves annexins, and a complex of tetraspanin CD63, syntenin-1 and ESCRT components. Therefore, tetraspanins and annexins might organize the spatial accumulation of HPV16-associated molecules and recruitment of cytoplasmic trafficking factors until capsid disassembly and L2 membrane insertion/penetration ensures virus interaction with cytoplasmic partner proteins and subsequent trafficking towards and into the nucleus.