Figure EV2. C‐ and N‐terminal NrCAM fragments stay attached after initial furin cleavage.
-
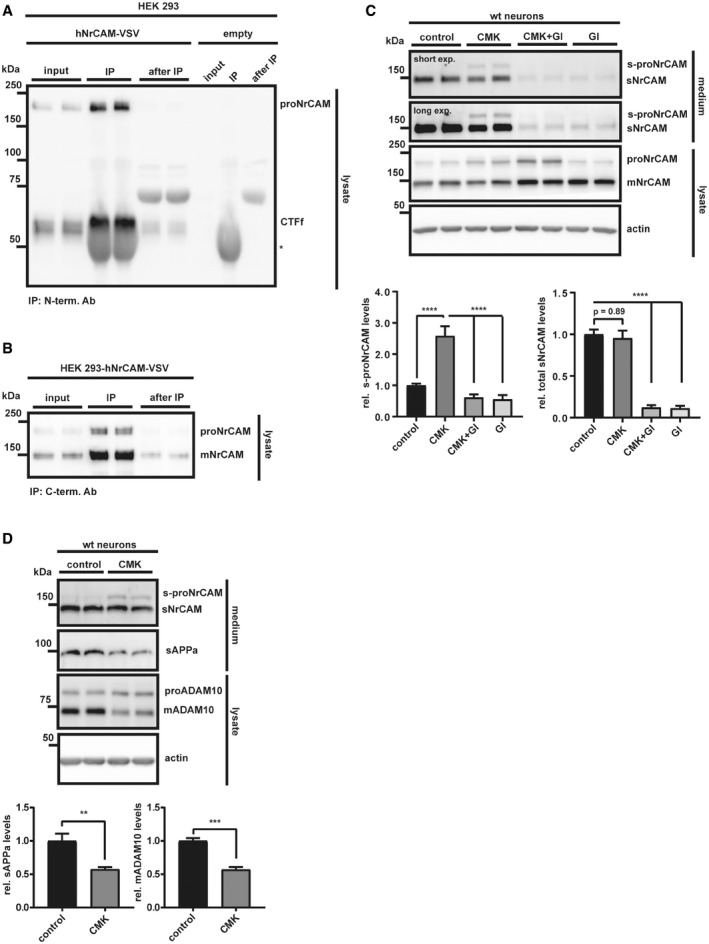
ACo‐immunoprecipitation of C‐ and N‐terminal NrCAM fragments in lysates of HEK 293 cells transfected with a C‐terminally VSV‐tagged NrCAM construct, or empty vector. Cells were lysed in CoIP buffer. IP was performed with an N‐terminal NrCAM antibody, detection with a C‐terminal VSV antibody. The CTFf (CTFfurin) band was running a little bit higher than in the input control, presumably because of the abundant IgG heavy chain, running right beneath it. Because of the lack of a suitable antibody, we were not able to detect the small fragment between the furin and the ADAM10 cleavage site. * indicates the IgG heavy chain.
-
BCo‐immunoprecipitation like in A, but the IP was done with a C‐terminal VSV antibody and the detection with an N‐terminal NrCAM antibody. NrCAM was detected as non‐cleaved proNrCAM and the furin‐cleaved mature mNrCAM.
-
CTo test whether NrCAM was sequentially cleaved by furin and then ADAM10, we treated primary neurons with the furin inhibitor dec‐CMK (50 μM), GI254023x (5 μM), or solvent for 48 h. Furin inhibition did not block NrCAM shedding, but caused the appearance of a 170 kDa band in the conditioned media, which was inhibited by GI254023x. In addition, dec‐CMK increased the 220 kDa proNrCAM (but not mNrCAM), which was even further increased by GI254023x. The levels of total sNrCAM remained unaffected by the furin inhibition. Densitometric quantifications of the Western blots are shown. One‐way ANOVA with post hoc Dunnett's test (****P < 0.0001, n = 6). Given are mean ± the standard error of the mean. The mean levels of solvent‐treated cells were set to 1.
-
DNeurons were treated with dec‐CMK or solvent like in (C). The small decrease in mADAM10 levels significantly reduced sAPPα, but not total sNrCAM release. Densitometric quantifications of the Western blots are shown. Two‐sided Student's t‐test (**P < 0.01; ***P < 0.001, n = 4). Given are mean ± the standard error of the mean. The mean levels of solvent‐treated cells were set to 1. Together, these results show that ADAM10 is not only able to cleave both mNrCAM and proNrCAM, but is also the protease to release sNrCAM into the conditioned media. Thus, NrCAM is firstly cleaved by furin and then by ADAM10, releasing sNrCAM. Representative Western blots are shown.
Source data are available online for this figure.
