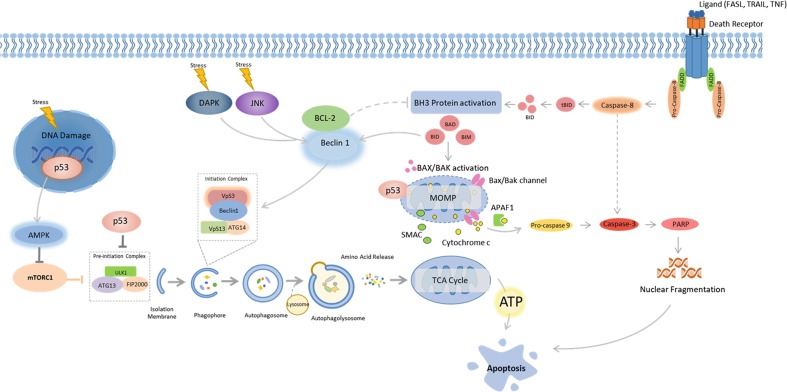
Fig. 2.
Cross-talk between autophagy and apoptosis. Autophagy and apoptosis share several inducers; p53, death-associated protein kinase (DAPK), JUN N-terminal kinase (JNK), BH3-only proteins BAD (BCL-2 antagonist of cell death), and BID (BH3-interacting domain death agonist). p53 translocates to the nucleus in response to excessive cellular stress and coordinates the expression of pro-autophagic proteins including AMPK. However, under prolonged stress p53 translocates to cyclophin D on the outer mitochondrial membrane, resulting in opening of the permeability transition pore (PTP) which promotes apoptosis. BH3-only proteins are conventional regulators of apoptosis, but are also capable of impairing the interaction between BCL-2 and Beclin 1. Unbound Beclin 1 is able to interact with VPS34 which initiates autophagy. BCL-2 loses its ability to repress apoptosis when not activated by Beclin 1. The Ser/Thr kinases DAPK and JNK contribute to a similar outcome on autophagy and apoptosis, where the BH3 binding domain of Beclin 1 is phosphorylated by DAPK, making binding of Beclin 1 to VPS34 possible, while JNK phosphorylates and inhibits BCL2, which activates Beclin 1