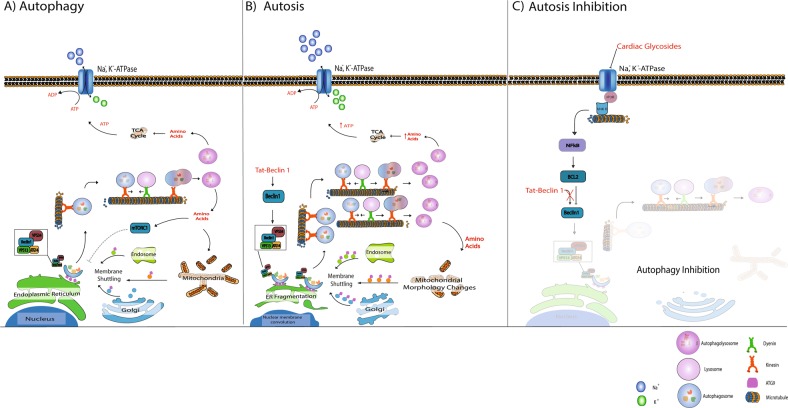
Fig. 3.
Autosis onset and inhibition. a Under normal conditions, autophagosome production is activated in response to nutrient deprivation. Autophagosome formation is highly dependent on ATG9 mediated membrane shuttling between the autophagosome formation site -usually close to the endoplasmic reticulum (ER)- and membrane sources such as the Golgi, mitochondria and endosomes. Once autophagosomes are matured, they are transported along the microtubule system by molecular motors such as dynein and kinesin to merge with acidic lysosomes, giving rise to autolysosomes which release amino acids. b Beclin 1 over-stimulation (through targeted peptides such as tat-Beclin1) results in a high demand for membrane material to meet the requirements of excessively high autophagosome production. This is, at least in part, the cause of ER fragmentation and mitochondrial morphology changes observed during autosis. It is plausible that ATP generated from excessively high autophagic activity is utilised by the Na, K, ATPase to compensate for the high ATP production, depriving the cell of energy over time. c Inhibiting the NKA with cardiac glycosides decreases both the demand for ATP and autophagy associated ATP production through the NKA-IP3R microdomain signalling, thereby rescuing the cell from autosis