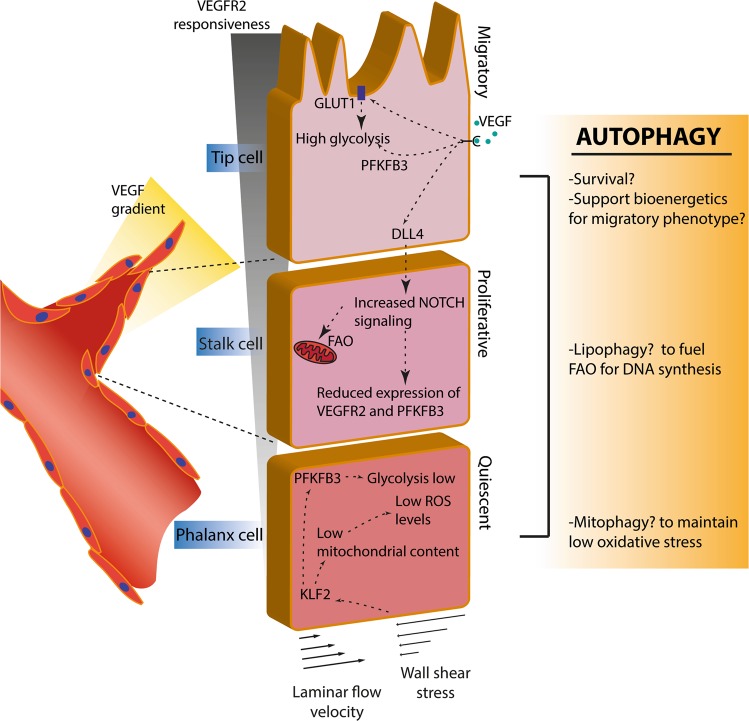
Fig. 3.
Regulation of distinct endothelial cell (EC) phenotypes during vessel sprouting. The vascular endothelium consists of three main EC subtypes with specialized morphological and functional features. In adults, ECs are mostly found in a non-proliferating, quiescent state (phalanx cells), yet are readily able to respond to external cues and initiate angiogenesis (the formation of new blood vessels from pre-existing vessels). This process entails the differentiation of ECs to guide the growing sprout or branch. Pro-angiogenic signals as vascular endothelial growth factor (VEGF) isoforms that bind to their receptor (VEGFR) stimulate EC migration, proliferation and sprouting. In ECs, VEGF binding to VEGFR2 signals to induce a sprouting migratory phenotype or a proliferating phenotype, referred to as tip or stalk cells, respectively. In brief, the activation of the VEGF/VEGFR2 axis in the tip cell induces the expression of the NOTCH ligand the Delta-like ligand (DLL)4 along with the upregulation of the rate-limiting glycolytic enzyme 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and glucose uptake, via the glucose transporter GLUT1. In the tip cell increased glycolysis fuels the cytoskeleton remodeling at lamellipodia and lobopodia, thereby supporting the migratory phenotype. DLL4 interaction with the NOTCH receptor on neighboring ECs leads to the proteolytic activation of the transcription factor Notch intracellular domain (NICD). NICD represses PFKFB3 and VEGFR2 expression while increasing fatty acid oxidation (FAO) that is required for DNA replication, thus supporting the proliferative stalk phenotype. In contrast, phalanx cells are kept quiescent due to laminar shear stress-induced Krüppel-like factor 2 (KLF2). KLF2 in turn, represses PFKFB3 expression, reduces proliferation, and causes reduction of mitochondrial content. Autophagy may be involved in the regulation of these subtypes through e.g. increasing the resistance to cell death upon e.g. hypoxic conditions, and by sustaining the high energy demand of tip and stalk cells through the modulation of metabolic pathways in these ECs. Stimulation of autophagy/mitophagy by laminar flow in phalanx cells maintains redox homeostasis to preserve EC quiescence