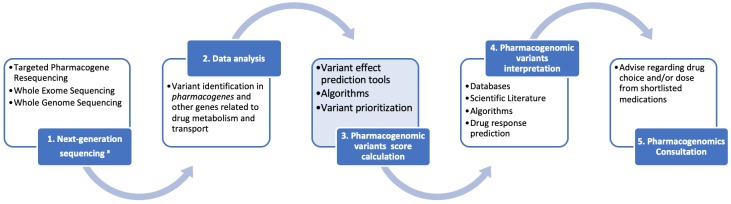
FIGURE 1.
A schematic representation for the clinical pharmacogenomics workflow described herein. We feel that the advent of next generation sequencing (NGS) will accelerate the clinical applications of pharmacogenomics through a series of reliable, cost-effective opportunities. Data collection and interpretation will benefit from the interplay of consortia and information technologies. Regulatory bodies will lead the way toward assay validation and accreditation, considering the difficulties of pharmacogenomics studies replication. Consultation, as the final step of our workflow, facilitates the bench-to-bed transition.