Figure 2.
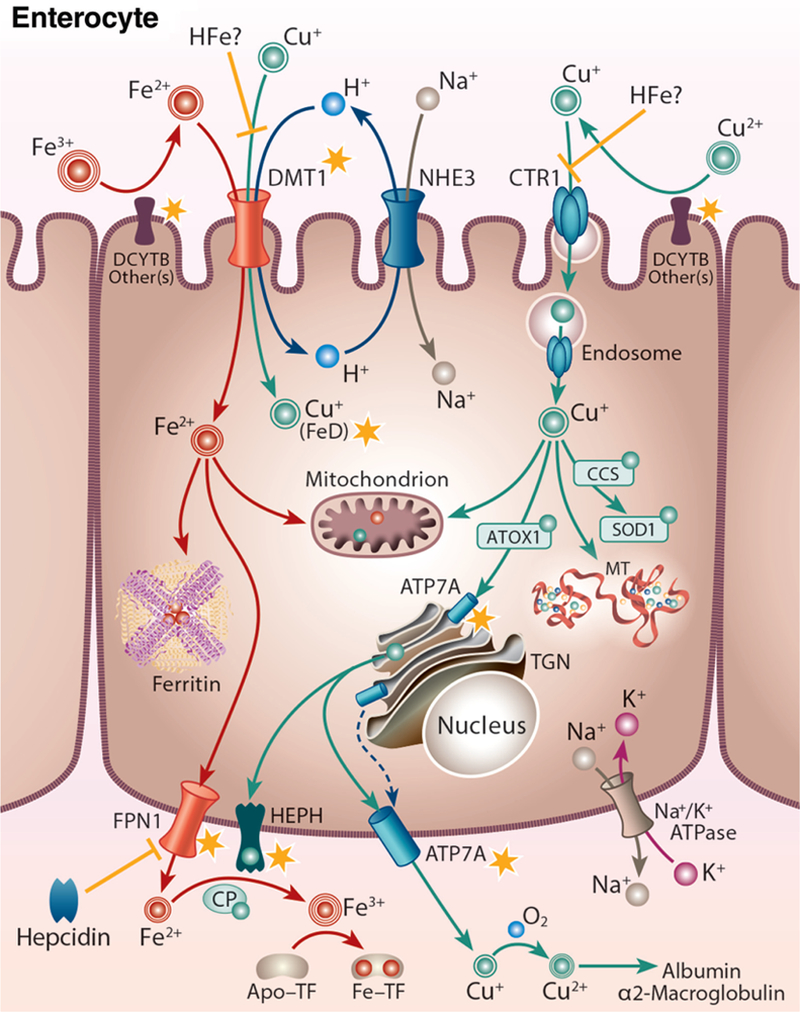
Iron-copper metabolism in a duodenal enterocyte, highlighting points of intersection between these two essential trace minerals. A duodenal enterocyte is depicted along with the proteins which mediate iron and copper absorption. Points where iron and copper metabolism intersect are demarcated by yellow stars. Both metals require reduction prior to absorption, which may be mediated by DCYTB and/or other reductases. Subsequently, iron is transported along with protons across the BBM by DMT1. The electrochemical proton gradient across the BBM that provides the driving force for ferrous iron transport is maintained via the action of a sodium-hydrogen antiporter (NHE3) and the Na+/K+ ATPase on the BLM. DMT1 may also transport copper during iron deficiency (FeD). High-iron (HFe) intake may block copper transport by DMT1 and/or CTR1, eventually leading to copper depletion. Cytosolic iron may be transported into mitochondria for metabolic use, stored in ferritin, or exported across the BLM by FPN1. FPN1 activity may be impacted by copper. Ferrous iron must then be oxidized by HEPH, CP, or other FOXs (not shown) to enable binding to TF in the interstitial fluids. After reduction, dietary copper is transported into enterocytes by CTR1 and is then distributed to various cellular locations by intracellular copper-binding proteins (i.e. chaperones). Excess copper may be stored in the cell by MT. Copper is pumped into the TGN by ATP7A, supporting cuproenzyme synthesis, or exported from the cell by ATP7A, which moves to the BLM when copper is in excess. ATP7A expression is strongly upregulated by iron depletion, suggesting that it (or copper) may positively influence iron metabolism in enterocytes. Copper is spontaneously oxidized by dissolved oxygen in the blood and then bound to mainly albumin and α2-macrogloubuoin in the portal blood and delivered to the liver.
