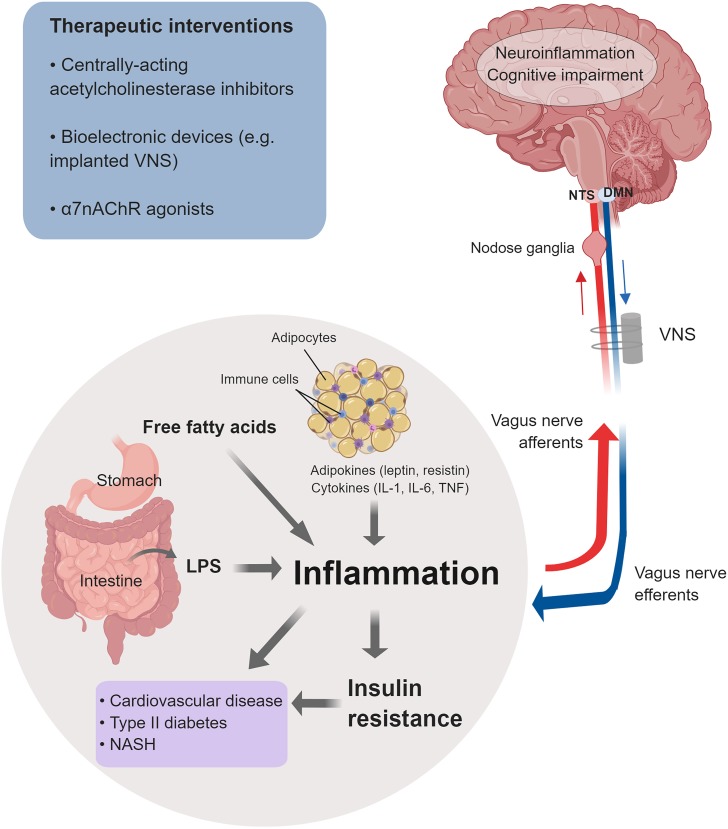
FIGURE 2.
Cholinergic control of inflammation and metabolic derangements in obesity-driven disorders. Inflammation in obesity is a major driver of insulin resistance and other metabolic derangements linked to an increased risk of cardiovascular disease, type 2 diabetes, NASH, and other disorders. Main factors that contribute to this low-grade chronic inflammation are: adipokines and cytokines released from enlarged adipocytes and immune cells infiltrating the expanded white adipose tissue; LPS as a result of microbiota alterations and increased intestinal permeability; and increased levels of free fatty acids. Inflammation and neuroinflammation in obesity are also linked to cognitive impairment. The vagus nerve provides a major conduit for communication between the brain and the periphery. Afferent (sensory) neurons residing in the nodose ganglia and terminating in the NTS detect alterations in peripheral inflammatory and metabolic molecules and communicate this information to the brain. Signaling through efferent cholinergic fibers that originate in the dorsal motor nucleus (DMN) plays an important role in controlling inflammation and metabolic derangements. Brain cholinergic signaling also is implicated in the regulation of cognition and controlling neuroinflammation. Brain and peripheral (vagus nerve) cholinergic signaling can be explored for therapeutic benefit in obesity and obesity-driven conditions. Preclinical and clinical studies have shown the efficacy of galantamine and other centrally acting acetylcholinesterase inhibitors, bioelectronic VNS, and α7nAChRs agonists in alleviating inflammation, metabolic derangements, neuroinflammation, and in improving cognition (See text for details). (This figure was created with BioRender.)