Figure 3.
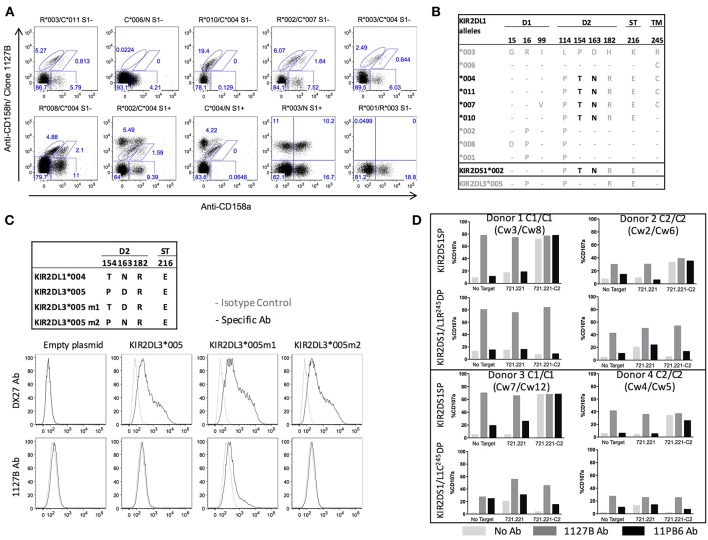
Differential binding and agonist effects of antibody 1127B to KIR2DL1 and KIR2DS1 allotypes. (A) Differential staining of KIR2DL1 allotypes by anti-CD158a and clone 1127B monoclonal antibodies. (B) Alignment of the amino acid sequences of KIR2DL1 allotypic variants. Dashes indicate identity with the consensus KIR2DL1*003 allotype. Structural domains are indicated: Ig like domains (D1 and D2), stem domain (ST) and transmembrane domain (TM). Alleles in black encode receptor variants recognized by both anti-CD158a and clone 1127B antibody staining. Candidate amino acid residues involved in recognition by clone 1127B are indicated in black. (C) Binding specificities by flow cytometry of anti-CD158b1/b2/j (DX27) and clone 1127B on HEK293 cells transfected with cDNA for KIR2DL3*005 or mutation variant exhibiting amino acid substitutions in D2, as indicated. (D) Cytotoxic response, as measured by CD107a mobilization, in KIR2DL1+KIR2DS1+ and KIR2DS1sp cells following co-culture with 721.221 and 721.221-HLA-Cw4 target cells without additional antibody or in the presence of 1127B or 11PB6 antibody. Results are shown from four different donors, representative of ten tested.