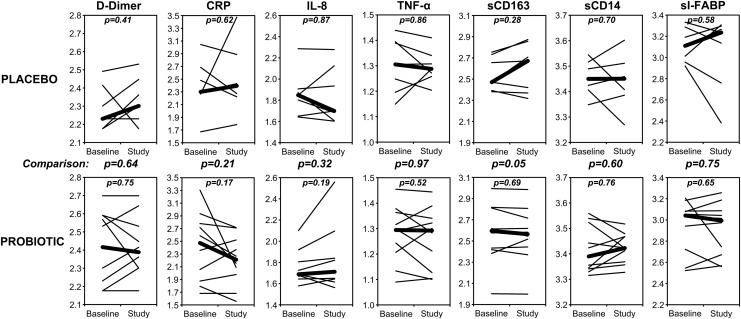
FIG. 3.
Levels of blood biomarkers during the study. For each individual, the blood concentrations of the indicated biomarkers are plotted for values at start to end of placebo/probiotic administration (thin lines), on log10 scales. Thick lines indicate the medians for each group. Not shown are lipopolysaccharide (LPS), interleukin (IL)-1β, and IL-6 because these were not detected (<0.05 EU/ml, <7.1 pg/ml, and <0.6 pg/ml, respectively). Changes in these markers within groups were not statistically significant. Comparisons of changes between groups approached significance (p<0.2) only for soluble (s)CD163 (p=0.05). Units were μg/ml for D-dimer, mg/dl for C-reactive protein (CRP), pg/ml for IL-8, pg/ml for tumor necrosis factor (TNF)-α, ng/ml for sCD163, μg/ml for sCD14, and ng/ml for serum intestinal-type fatty acid binding protein (sI-FABP).