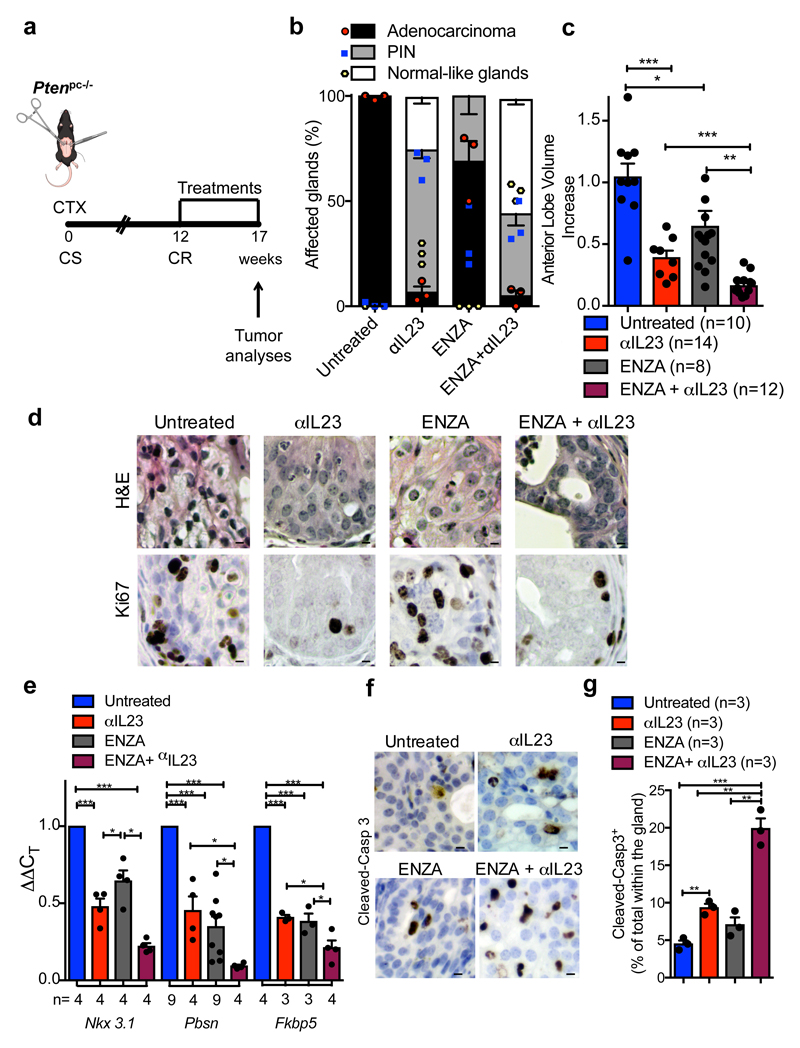
Figure 4. IL23 inhibition improves enzalutamide efficacy in vivo.
a, CR Ptenpc-/- mice (12 weeks after castration) were randomly enrolled in the preclinical trial. Treatments: Isotype control (Untreated), anti-IL23 antibody (αIL23; 100ng/per mouse i.p. weekly), Enzalutamide (ENZA, 30 mg/kg/day administered daily by oral gavage on a Monday to Friday schedule) and Enzalutamide in combination with anti-IL23 antibody (ENZA+αIL23). b, Histological score. n=3 biological independent animals. Statistical analyses (Two-way ANOVA): p<0.001. c, Fold increase of the prostate anterior lobe volume (fold change compared with untreated group). d, Representative H&E and Ki-67 staining in the tumors at completion of the study. Scale Bar 50 μM. e, qRT-PCR analyses of the indicated genes in the prostate tumors of CTX-Ptenpc-/- mice at completion of the preclinical trial. Statistical analyses (two-sided Paired t test): *P <0.05; ***P <0.001. f, Representative cleaved-Caspase3 staining in the tumors after one week of treatments. Scale Bar 50 μM. g, Quantification of cleaved-Casp 3 (percentage of total within the glands). d, f, Data are representative of at least in three biological independent animals. b, g, Data are reported as mean ± SEM of one tumor per mouse (mean of three sections per mouse, ≥ 3 fields per section). b,c,e,g Data are reported as mean ± SEM. Specific n values of biological independent animals are shown in c, e, g. c, g, Statistical analyses (Unpaired two-sided t-test): ns, not significant; *P <0.05; **P <0.01; ***P <0.001.