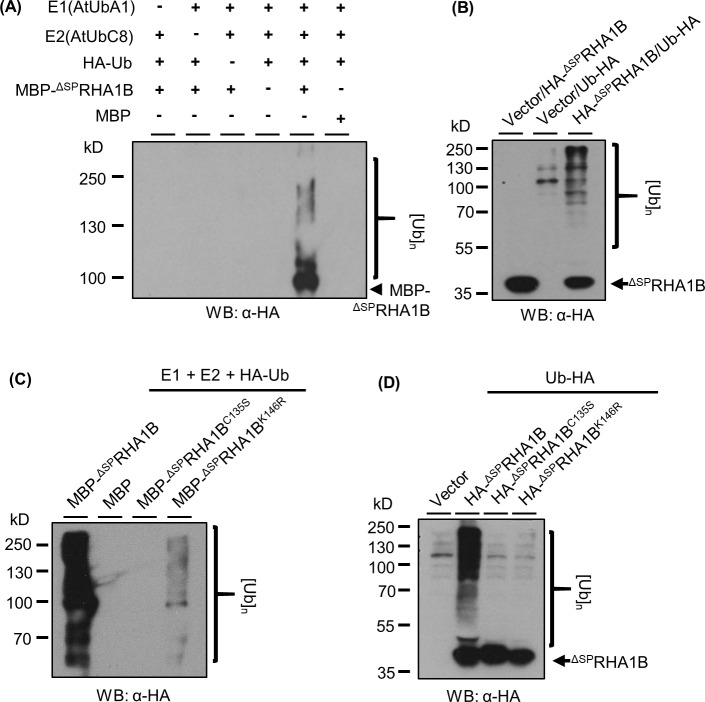
Fig 2. RHA1B is an active E3 ubiquitin ligase.
(A) in vitro self-ubiquitination of RHA1B. Self-polyubiquitination of RHA1B was determined by Western blotting (WB) using anti-HA antibody. (B) RHA1B enhances ubiquitination in planta. Ubiquitination level in agro-infiltrated N. benthamiana leaves was determined through WB using anti-HA antibody. (C/D) Zn-finger motif and the Lys135 residue are essential for the intrinsic E3 activity of RHA1B. Similar in vitro ubiquitination (C) and in vivo ubiquitination assay (D) including the ΔSPRHA1BC135S and ΔSPRHA1BK146R mutants were conducted as in (A) and (B), respectively. (A-D) Experiments were repeated at least three times with similar results.