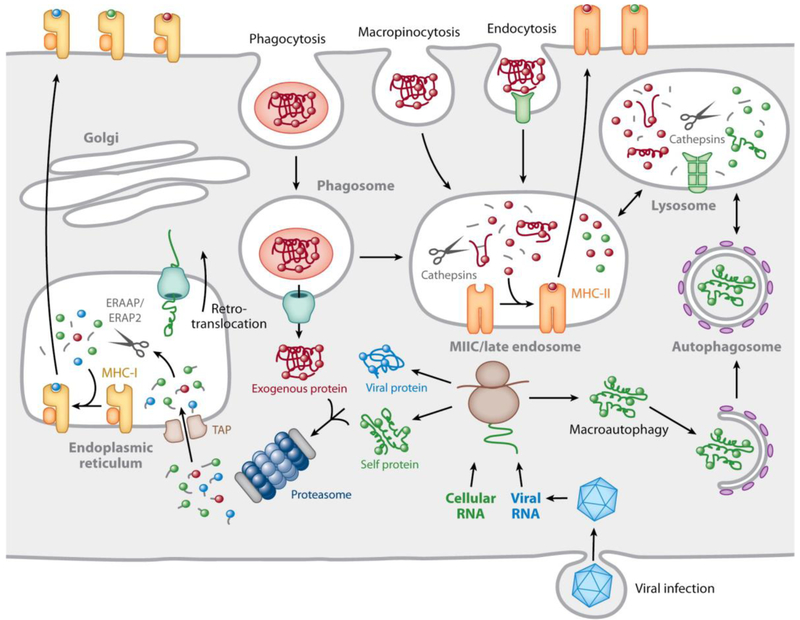
Fig. 1. Mechanisms underlying MHC-II-restricted antigen processing.
MHC-II αβ dimers associate with trimers of the invariant chain (labeled I) in the ER. When the nonameric complex is completely assembled it leaves the ER and moves through the Golgi apparatus before being diverted into the endocytic pathway by a C-terminal cytoplasmic motif in the invariant chain. Late in the endocytic pathway, in the MHC-II compartment or MIIC, exposure to an acidic environment combined with a collection of proteases known as cathepsins results in invariant chain proteolysis, leaving the Class II-associated Invariant chain Peptide (CLIP) in the MHC-II binding groove. Peptides derived from exogenous proteins internalized by endocytosis, micropinocytosis, or phagocytosis, or introduced from the cytosol by autophagy, are also proteolyzed, producing a range of peptides. A subset of these peptides has appropriate sequences for binding to the specific MHC-II alleles expressed by the cell. However, binding does not occur spontaneously. The MHC-II molecule interacts with a homologous non-peptide binding αβ dimer, DM, which is resident in the MIIC, which allosterically modifies the MHC-II binding groove to induce CLIP release and exchange for locally generated peptides. Peptides of sufficiently high affinity avoid subsequent DM-induced release and are expressed on the plasma membrane. A second MHC-II homologue, DO, can bind to DM in the ER and DM/DO complexes also localize to the MIIC but are inactive in inducing CLIP release. DO serves to modulate DM-induced CLIP release and peptide exchange. (reproduced from Blum et al., 2013).