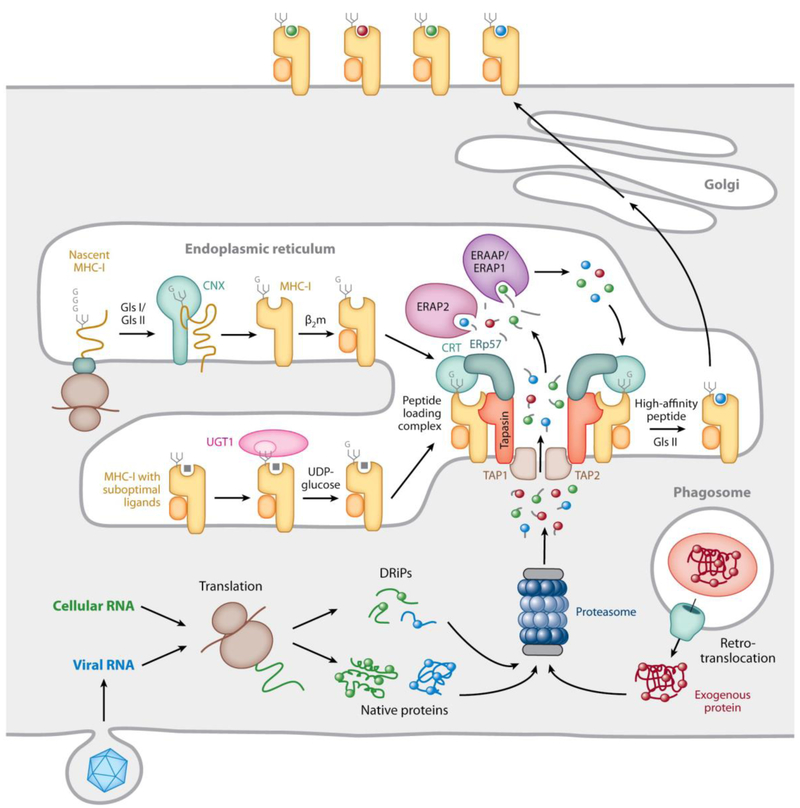
Fig. 3. Mechanisms underlying MHC-I-restricted antigen processing.
MHC-I-β2m dimers assemble in the ER with the help of the lectin chaperone calnexin (CNX in the figure) before incorporation into the peptide loading complex (PLC). This involves non-covalent interactions of the MHC-I-β2m dimer with calreticulin (CRT in the figure), via a monoglucosylated N-linked glycan at position 86 of the MHC-I heavy chain, and with tapasin, two copies of which are non-covalently associated with the TAP1/TAP2 heterodimeric transporter. The MHC-I/calreticulin/tapasin interactions are cooperatively further stabilized by calreticulin association with ERp57, which is itself disulfide-linked to tapasin (see Fig. 4). Peptides generated by proteasomal degradation from cellular or pathogen-derived cytosolic proteins, perhaps prematurely terminated or otherwise defective ribosomal products (DRiPs), are translocated via TAP into the ER, where they can be N-terminally trimmed by the ER aminopeptidases ERAP1 (ERAAP in mice) and ERAP2. Tapasin is assumed to maintain the MHC-I binding groove in an open, peptide-receptive state within the PLC. Peptides of the appropriate sequence and length can bind to the MHC-I molecules and if they are of a sufficiently high affinity the peptide-occupied MHC-I-β2m dimers may dissociate from the PLC. The terminal glucose of the N-linked glycan can be removed by the enzyme glucosidase II and if the peptide is suboptimal, i.e. unable to maintain the properly folded conformation of the MHC-I molecule, the glycan can be re-glucosylated by the enzyme UDP-glucose glycoprotein transferase-1 (UGT1), allowing the MHC-I-β2m dimers to maintain interaction with the PLC or to re-enter it. The end products of these complex quality control processes are stable, surface expressed MHC-I-peptide complexes. Illustrated in the lower left of the figure is the process thought to initiate the cytosolic pathway of cross-presentation: proteins internalized by phagocytosis are extruded into the cytosol, where they meet the same proteolytic fate as endogenously synthesized proteins. (reproduced from Blum et al., 2013).