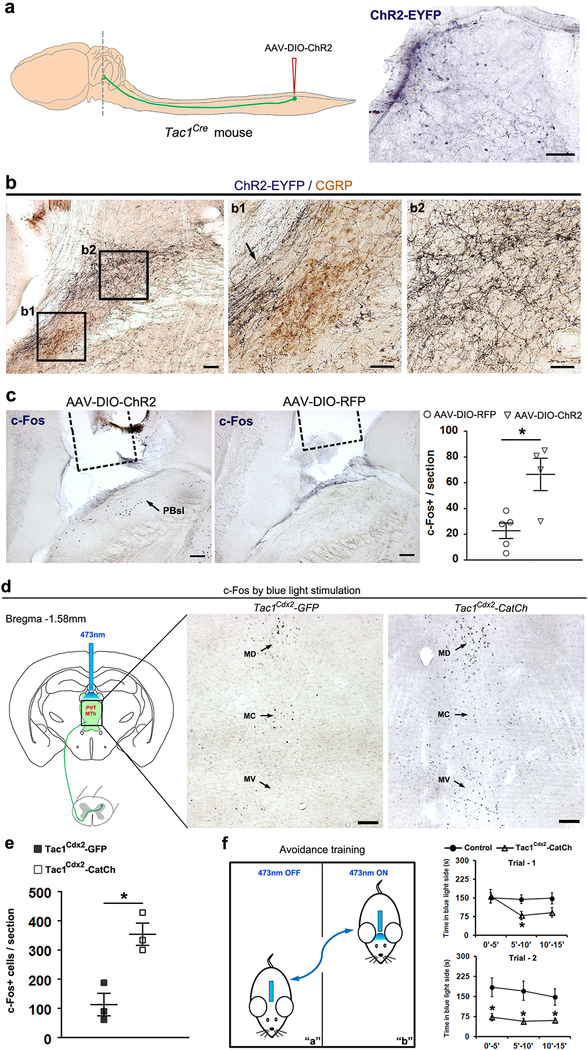
Extended Data Fig. 9. Optogenetic activation of Tac1Cre-derived ascending terminals around the parabrachial nucleus (PBN, a-c) or medial thalamus (d-f).
a-c, Viral infection in lumbar spinal cord Tac1Cre+ neurons and subsequent optogenetic activation of the central terminals in the PBN area. a, The AAV-DIO-ChR2 virus, which drove the expression of the fusion ChR2-EYFP protein in a Cre-dependent manner, was injected into the lumbar dorsal horn of Tac1Cre mouse, and the ascending ChR2-EYFP+ terminals around the PBN (dash line) were visualized by a GFP antibody. These mice are referred to as Tac1Cre-ChR2 mice. Right, immunostaining shows ChR2-EYFP fusion protein expression in the lumbar spinal cord. b, Representative images showing double-color immunostaining, revealing the ascending projections to the PBN at Bregma −5.02 mm. Tac1Cre-ChR2-EYFP+ terminals (dark blue) were co-stained with CGRP (brown). Note that Tac1Cre-ChR2-EYFP+ fibers pass through a region (b1, arrow) lateral to the CGRP+ external lateral PBN (b1, PBel, brown), and terminated densely in the superior lateral PBN (b2, PBsl). c, Representative images showing Blue light stimulation-induced c-Fos expression in PBsl of Tac1Cre-ChR2 mice, with much fewer c-Fos+ neurons following blue light stimulation in control Tac1Cre-RFP mice, in which viral injection drove the expression of RFP but not ChR2 (ChR2 mice, n = 4; RFP control mice, n = 5; two-sided t-test, P = 0.012). Dashed lines indicate the location of the implanted optic fiber in the region right above the PBN. d-f, Optogenetic experiments for spinal Tac1 neurons projected to medial thalamic nuclei (MD, MC and MV). d,e, Generation of the intersectional Tac1Cdx2-CatCh+ mice was described in Extended Data Figure 2b. The optic fiber was implanted above the medial thalamic complex (left scheme). Neuronal activation by blue light stimulation was indicated by the increase of c-Fos+ cells in Tac1Cdx2-CatCh mice in comparison with Tac1Cdx2-GFP mice, as shown by representative images and quantitative analyses (Tac1Cdx2-CatCh: n = 3; Tac1Cdx2-GFP: n = 3; two-sided t-test, P = 0.011). f, The Tac1Cdx2-CatCh mice showed progressive avoidance to the blue light-paired chamber during two 15 min-training trials conducted at two consecutive days (see Methods for detail). Two-way ANOVA plus post hoc Bonferroni’s t-test showed a progressive avoidance to the paired chamber (Tac1Cdx2-CatCh mice, n = 10; Tac1Cdx2-GFP control mice, n = 11; trial 1, significant interaction, F(2,38) = 5.067, P = 0.011; trial 2, significant genotype effect, F(1,19) = 6.825, P = 0.017, no interaction, F(2,38) = 0.73, P = 0.489).