Figure 1. STIM1 stoichiometry in the inactive and active states.
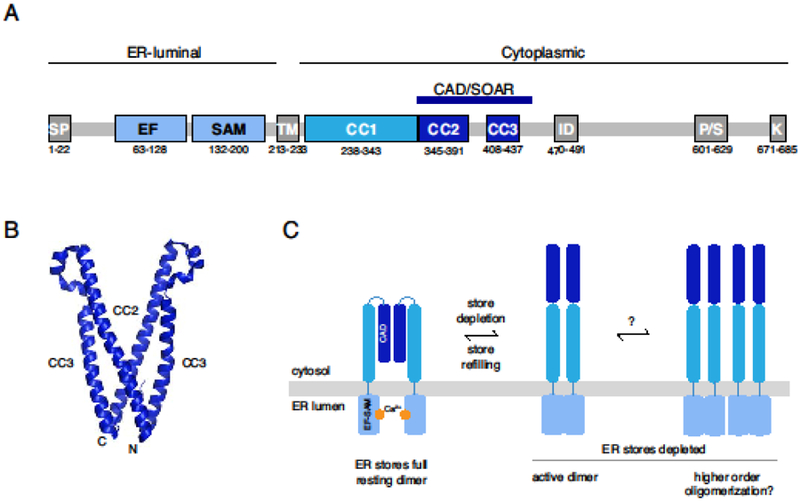
(A) Schematic of STIM1 domain architecture showing the signal peptide (SP) and the EF-SAM, ER transmembrane (TM), coiled-coil (CC1-CC3), CAD/SOAR, inactivation (ID), proline/serine-rich (P/S), and polybasic (K) domains. Numbered residues denote the domain boundaries for human STIM1. For further details see [2]. (B) Crystal structure of the dimeric CAD/SOAR (3TEQ.pdb) [13]. N- and C-terminal ends of the front subunit are indicated. (C) In the resting state, STIM1 is dimerized through interactions of the CAD domain, and the EF-SAM domains are separated in the ER lumen. After store depletion, the EF-SAM domains associate (left), extending the CAD towards the PM, and may also form higher-order oligomers through association of multiple STIM1 dimers (right). Regions C-terminal to the CAD have been omitted for clarity.
