Abstract
Nonalcoholic steatohepatitis (NASH) is a rapidly growing cause of chronic liver damage, cirrhosis, and hepatocellular carcinoma (HCC). How fatty liver pathogenesis is subject to epigenetic regulation is unknown. We hypothesized that chromatin remodeling is important for the pathogenesis of fatty liver disease. ARID1A, a DNA-binding component of the SWI/SNF ATP-dependent chromatin-remodeling complex, contributes to nucleosome repositioning and access by transcriptional regulators. Liver-specific deletion of Arid1a (Arid1a LKO) caused the development of age-dependent fatty liver disease in mice. Transcriptome analysis revealed upregulation of lipogenesis and down-regulation of fatty acid oxidation genes. As evidence of direct regulation, ARID1A demonstrated direct binding to the promoters of many of these differentially regulated genes. Additionally, Arid1a LKO mice were more susceptible to high-fat diet-induced liver steatosis and fibrosis. We deleted Pten in combination with Arid1a to synergistically drive fatty liver progression. Inhibition of lipogenesis using CAT-2003, a potent SREBP inhibitor, mediated improvements in markers of fatty liver disease progression in this Arid1a/Pten double knockout model.
Conclusion:
ARID1A plays a role in the epigenetic regulation of hepatic lipid homeostasis, and its suppression contributes to fatty liver pathogenesis. Combined Arid1a and Pten deletion shows accelerated fatty liver disease progression and is a useful mouse model for studying new therapeutic strategies for NASH.
Keywords: NAFLD, SREBP, hepatocellular carcinoma, NASH, liver
The liver plays a central role in governing the traffic and utilization of lipid molecules, and loss of hepatic lipid homeostasis leads to fatty liver disease and systemic dyslipidemia. Non-alcoholic fatty liver disease (NAFLD), defined as hepatic steatosis in the absence of significant alcohol usage, is a growing epidemiologic problem, with a worldwide prevalence of 20% (1). In the United States, approximately one-third of the population shows signs of steatosis (2), and NAFLD accounts for roughly 75% of chronic liver disease in the United States (3). These figures have risen dramatically in the past 20 years, along with rates of obesity, type II diabetes, and hypertension (3). The pathogenic progression of fatty liver disease is variable and complex; while many individuals are asymptomatic and only detectable by liver biopsy or ultrasound, biopsy series estimate that 3–5% of the population has nonalcoholic steatohepatitis (NASH), an advanced stage of fatty liver disease that involves inflammatory infiltration and recurrent liver damage (1). The most severe cases may progress to cirrhosis, hepatocellular carcinoma (HCC), and ultimately death. This progression can be unpredictable, with some patients spending years cycling between simple steatosis and inflammation while others quickly advance to end-stage disease within 5–6 years (4). The factors that contribute to this heterogeneity of disease are extensive, including diet and other environmental factors, intestinal microbiota, and genetics.
The contributions of epigenetic regulation to the pathogenesis of fatty liver disease are currently poorly understood. Several studies have demonstrated that there are differences in DNA methylation between patients with mild and advanced NAFLD that appear to affect pathways related to DNA repair, metabolism, and fibrosis (5, 6). Mann et al. (7) have shown that MeCP2-mediated chromatin remodeling via the PRC1 Polycomb complex may promote hepatic fibrosis by repressing the transcription of PPARγ, an anti-fibrotic and adipogenic nuclear receptor (8, 9). Other work by Kim et al. (10) demonstrate that overnutrition leads to the activation of steatotic target genes of PPARγ2 via association with the histone methyltransferase MLL4, providing an important link between diet and epigenetic factors.
The SWI/SNF family of chromatin remodeling complexes is an attractive target for possible epigenetic regulation of liver metabolic homeostasis. The complex uses energy derived from ATP to shift DNA along nucleosomes, allowing for the opening of chromatin and altered access by transcriptional machinery (11). The core ATPase (BRM or BRG1) is guided to its targets by several DNA-binding subunits, one of which is ARID1A (12). Previous work by Sun et al. has shown that suppression of Arid1a within the murine liver improves regenerative capacity (13) and that Arid1a has context-dependent tumor suppressive and oncogenic effects in liver cancer (14). Fang et. al. have previously shown that liver-specific loss of Arid1a leads to steatohepatitis and increased levels of TNF-α and IL-6, but additional mechanisms and therapeutic approaches were not evaluated (15). Here, we sought to further explore the role of ARID1A and the SWI/SNF complex in the maintenance of lipid homeostasis by examining the lipid transcriptome in greater detail. We also combined the Arid1a liver knockout model with an adenovirus-Cas9 mediated Pten knockout model to accelerate fatty liver development, allowing for more efficient examination of steatosis-mediated end-stage liver disease. Finally, we examined how small-molecule inhibition of downstream targets of ARID1A may help to maintain lipid homeostasis, providing a possible therapeutic target for this challenging disease.
Experimental Procedures
Mouse Handling and Treatments
All mice were kept in a pathogen-free barrier facility and handled in accordance with the guidelines of the Institutional Animal Care and Use Committee at UT Southwestern. In Arid1a floxed mice, induced deletions between the two loxP sites produce cells lacking exon 8 of Arid1a (see Supplemental Information for a list of primers), which create a frameshift mutation and induce nonsense-mediated decay in the resulting transcript (16). Mice were allowed to feed ad libitum unless specifically noted otherwise. These mice were a mix of C57/B6 and 129 strains. All experiments were done in an age- and sex-controlled fashion unless otherwise noted in the figure legends. Primers used for genotyping are documented in Table S1.
Human Tissue Analysis
Six normal liver tissues and six fatty liver tissues from liver donors were enrolled in our study to validate the expression levels of related genes. All tissues were histologically diagnosed. Prior patient consent and ethical approval from the ethics committee of the First Affiliated Hospital, Sun Yat-sen were obtained. All methods were performed in accordance with the ethics guidelines and regulations. Total RNA from tissues was isolated with TRIzol reagent (Invitrogen) in accordance with the manufacturer’s protocol. cDNA synthesis was performed according to standard procedures using primeScript RT Master kit (Takara Bio Inc.). Quantitative RT-PCR was performed by SYBR Green quantitative PCR kit (Takara Bio Inc.) using the LC480 Real-Time PCR System (Roche). The primer sequences are provided in Table S1. β-tubulin was used as a reference gene. Every test was repeated by three replicates. Data was presented as mean ± SEM and Student’s t-test was used to evaluate statistical difference.
Histology and Immunohistochemistry
Tissue samples were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin. Primary antibodies used were PTEN (Cell Signaling Technology, #9559S). Blocking was performed with the Avidin/Biotin blocking kit (Vector Laboratories, SP-2001), and detection was performed with the Elite ABC Kit (Vector Laboratories, PK-6101) and ImmPACT DAB kit (Vector Laboratories, SK-4105), followed by Hematoxylin Solution counterstaining (Vector Laboratories, H-3404). For Oil Red O staining, frozen mouse liver tissues were cut at 6 μm, mounted on slides, air dried for 1 hr before the sections were fixed with 10% formalin for 10 min. The slides were then rinsed with PBS, air dried and then placed in 100% propylene glycol for 2 min. Next the slides were stained in 0.5% Oil Red O solution in propylene glycol for 30 min before transferred to an 85% propylene glycol solution for 1 min. Finally, the slides were rinsed in distilled water for 2 changes, and processed for hematoxylin counter staining. For Sirius Red staining, paraffin-embedded liver sections were deparaffinized and rehydrated before incubated with picro-sirius red solution (1.2%) for 60 minutes. Slides were then quickly rinsed with water then dehydrated with rinsing sequentially in 70% EtOH, 100% EtOH, and xylene before mounting.
Liver Function Tests and Hepatic Lipid Composition
Blood samples were taken retro-orbitally in heparinized tubes and centrifuged to separate out plasma from whole blood. Liver samples for hepatic lipid composition were collected immediately after mouse sacrifice and snap-frozen in liquid nitrogen. Tests were run by the UT Southwestern Metabolic Phenotyping Core using a VITROS 250 Chemistry Analyzer and a BioTek SynergyMX Microplate Reader, respectively.
Pathological Grading
H&E histological slides were graded by P.G., a clinical pathologist with expertise in chronic liver disease and cancer. She was blinded to the identity of the samples and evaluated them in accordance with the NAFLD activity scoring (NAS) system (17), which assigns values to degree of steatosis, inflammation, and hepatocyte ballooning as well as an overall NASH score.
High Fat Diet
Diet used was 60% kcal from fat (Research Diets, Inc. D12492). Mice were allowed to feed freely and weight of intake was measured weekly.
Intraperitoneal Glucose Tolerance Testing (IPGTT)
Study mice were fasted overnight (16 hours) before IPGTT, tail blood glucose levels were measured using Bayer Contour glucometer kit at baseline (t = 0) and 15, 30, 60, 120 minutes after mice received an i.p. injection of glucose (2g/kg of body weight).
Body Composition Testing
Body fat and lean mass for live study mice were measured using an EchoMRI-100 body Composition Analyzer (Echo Medical Systems, LLC).
RNA Extraction and RT-qPCR
Total RNA was isolated from liver tissue using Trizol reagent (Invitrogen, 15596018) and the RNeasy Mini Kit (Qiagen, 74104). cDNA synthesis was performed using 1μg of total RNA using the iScript cDNA synthesis kit (Bio-Rad, 1708891). mRNA expression was measured using the ΔΔCt method or using standard curves (where noted). Primers used are documented in Table S1.
RNA-sequencing
RNA from four WT and four Arid1a LKO livers from mice at three months of age were purified with the QIAGEN miRNeasy Mini Kit. Illumina or NuGEN libraries were made with these RNAs. These indexed libraries were multiplexed in a single flow cell lane and received 75 base single-end sequencing on an Illumina HiSeq 2500 using 50SR SBS v3 reagents at the UT Southwestern McDermott Center Sequencing Core or a NextSeq 500 using the High Output Kit v2 (75 cycles) at the CRI Sequencing Facility. Sequence reads were aligned to mouse genome version mm9 using TopHat, and differential expression analysis was performed using edgeR.
Gene Set Enrichment Analysis (GSEA)
GSEA was used to identify gene sets and pathways associated with gene expression data obtained from RNA-sequencing as above. GSEA input was a list of significantly differentially expressed genes between WT and Arid1a LKO livers as determined by edgeR. Each gene in the list was weighted by its log fold change in expression. Please see http://software.broadinstitute.org/gsea/index.jsp as well as Mootha et al and Subramanian et al for additional details (18, 19)
ChIP-seq
ChIP-seq was performed as previously described using a V5 tag in-frame with the endogenous coding region of Arid1a (13).
Western blotting
Mouse liver tissues were homogenized and lysed in T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific). Western blots were performed in the standard fashion using Mini-PROTEAN 4–20% SDS-PAGE gels and the Trans-Blot Turbo Transfer System (Bio-Rad). The following antibodies were used: anti-SREBP1 mouse antibody (SCBT, sc-13551), anti-FAS mouse antibody (SCBT, sc-48357), anti-FABP5 goat antibody (R&D Systems, AF1476), Anti-rabbit IgG, HRP-linked Antibody (Cell signaling, #7074), Anti-mouse IgG, HRP-linked Antibody (Cell signaling, #7076), and donkey anti-goat IgG, HRP-linked antibody (SCBT, sc-2020).
shRNA knockdown experiments
293T cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Sigma) and were maintained at 37°C in a humidified atmosphere with 5% CO2. For lentivirus production, 3.5 μg of the appropriate plasmid, 2.7 μg of gag-pol, and 0.5 μg of VSVg were transfected into 293T cells cultured at 50% confluence in 10cm plates using Lipofectamine 3000 (Invitrogen). Fresh medium was replaced 24 hours after transfection. The viral supernatants were harvested 72 hours after transfected and were centrifuged at 2000 rpm for 15 minutes to remove cell debris. 3×105 HuH-7 or HepG2 cells were plated into 6-well plates the day before infection. When the cells reached ~70% confluence, 0.5 mL of viral supernatant was added and removed 24 hours later. After 1 week puromycin selection, 2.5×104 cells stably expressing either shRNA against Arid1a (sequence: ATTGAACCAGATAGATCAGGC) or GFP were plated in triplicates in 12-well plates and cell numbers were counted for 3 days to test cell proliferation.
Adenoviral experiments
Ad-Cas9-sgPten was generated using a Vector Biolabs backbone with guide sequence AGATCGTTAGCAGAAACAAA obtained from Xue et al (20). 100 μL of virus at 1X109 PFU/μL was retro-orbitally injected to mediate PTEN deletion.
CAT-2003 Experiments
CAT-2003 was supplemented in the diet (0.75% weight/weight) and was given to study mice either immediately after Ad-Cas9-sgPten virus injection (prophylactic model) or 14wks after mice received virus injection (therapeutic model). For the Arid1aFl/Fl; PtenFl/Fl; Alb-Cre model, CAT-2003 was supplemented beginning approximately 6 weeks of age.
Statistical Analysis
Data shown is mean ± SEM unless otherwise indicated in the text. Analysis between two groups was performed using Student’s t-test; analysis between multiple groups was performed using two-way ANOVA with Tukey’s multiple comparison testing. Analysis for NAS scoring was performed using Mann-Whitney testing. p-values are reported as *p < 0.05, **p < 0.01, ***p <0.001, ****p < 0.0001 unless otherwise noted.
Results
Arid1a deficient livers are more susceptible to fatty liver disease
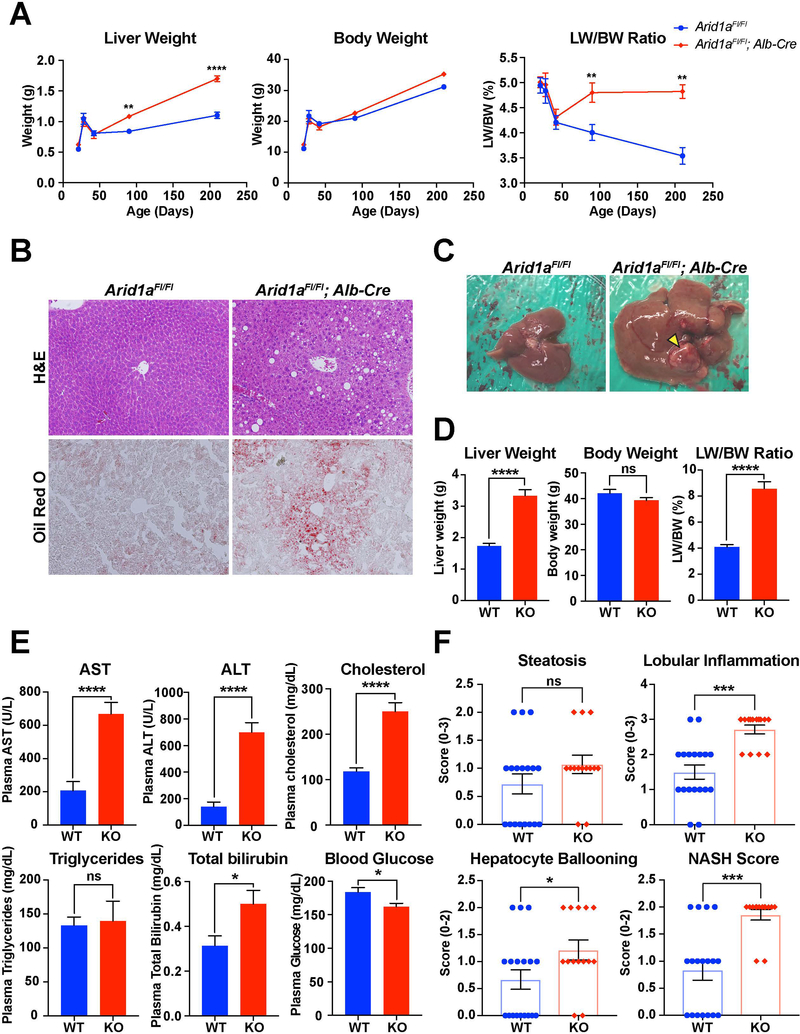
To determine the relationship between Arid1a and fatty liver disease, we characterized mice with liver-specific deletion of Arid1a generated with Albumin-Cre (Arid1a LKO). Arid1a LKO mice are healthy and their livers are grossly and histologically indistinguishable from wild-type (WT) littermates up to three months of age (Fig. S1A). After this time point, however, Arid1a-deficient livers gradually enlarged in comparison with WT, with no associated change in body weight (Fig. 1A). Histological examination at six months of age revealed that there was increased fatty droplet size and Oil red O staining, indicative of increased steatosis (Fig. 1B). Aged 14–16 month old Arid1a LKO mice showed similar changes in liver and body weight (Fig. 1C,D), and serum values taken from these mice showed increases in AST, ALT, total bilirubin, and cholesterol, suggestive of increased liver damage and steatosis as compared to WT controls (Fig. 1E). Intriguingly, there was no difference in serum triglycerides and random blood glucose levels were significantly lower in Arid1a LKO mice. To better characterize the extent of disease progression, liver histology from these mice was graded using the NAFLD Activity Score (NAS), which measures degree of steatosis, lobular inflammation, and hepatocyte ballooning (17). Arid1a LKO mice had significantly higher scores in the categories of lobular inflammation, hepatocyte ballooning, and the overall NASH diagnosis score (Fig. 1F, S1B).
Figure 1. Arid1a LKO mice showed increased hepatic fat infiltration at baseline.
A. Comparison of body weight, liver weight, and LW/BW ratio over the first six months of life. Number of mice at each time point is as follows: 21 days (n = 3,4), 28 days (n = 3,4), 42 days (n = 6,5), 90 days (n = 5,5), 210 days (n = 3,2).
B. H&E and Oil Red staining of WT and LKO livers at 6 months of age.
C. Gross images of WT and LKO livers at 15–17 months of age. Yellow arrowhead indicates tumor.
D. Body weight, liver weight and LW/BW ratio of WT and LKO mice aged to 15–17 months (n = 18, 14)
E. Notable serum markers comparing WT and LKO mice at 15–17 months of age (n = 18, 14).
F. NAS scores for WT and LKO mice at 15–17 months of age (n = 18, 14)
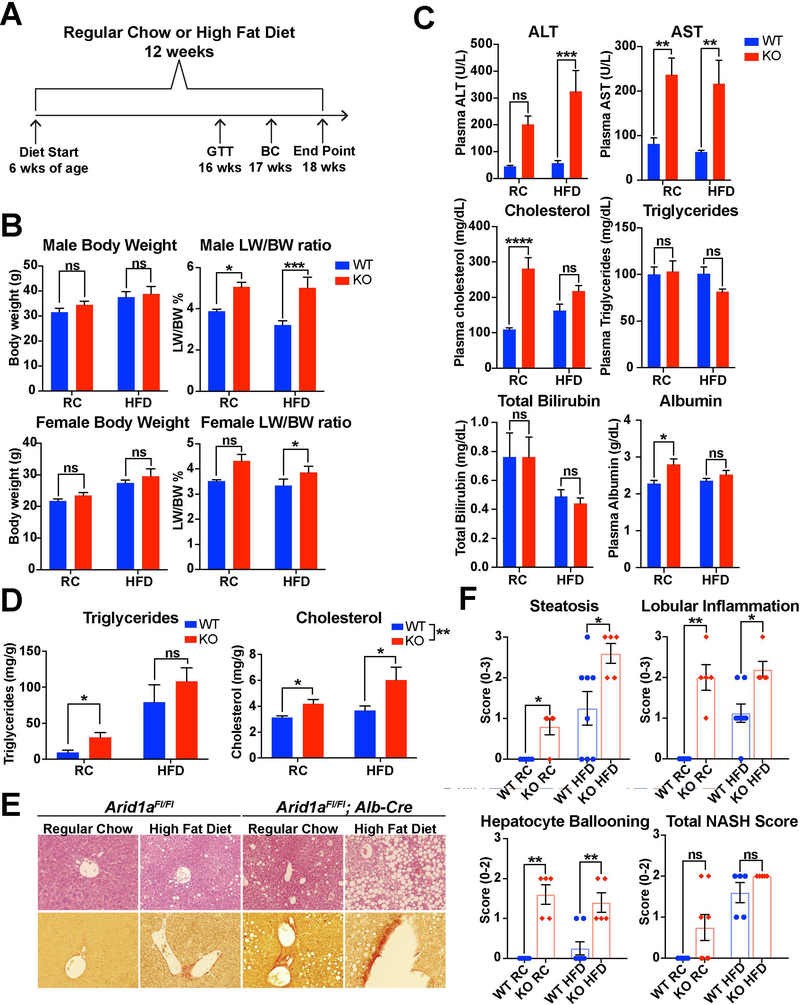
To determine if Arid1a deficiency had an impact on nutritional susceptibility to fatty liver disease, we fed both WT and Arid1a LKO mice a high fat diet (HFD) composed of 60% of calories from fat over a period of 12 weeks (Fig. 2A). Compared to their WT counterparts, LKO mice showed an increase in both liver weight and liver/body weight ratios in both male and female mice with no significant increase in body weight (Fig. 2B). This was supported by comparison of gross liver specimens, which exhibited enlargement as well as a pale discoloration as compared to WT controls (Fig. S1C). As seen in the Arid1a LKO mice receiving a regular diet, AST, ALT, cholesterol, and total bilirubin were significantly elevated as compared to WT, while serum triglycerides were unchanged with respect to diet or genotype (Fig. 2C). Hepatic triglycerides and cholesterol were both elevated in Arid1a LKO mice on a regular diet, with a marked additional increase in cholesterol levels on HFD (Fig. 2D). This aligns with previous research that has shown that the transition from NAFLD to NASH is marked by increased cholesterol synthesis without further increases in de novo lipogenesis (21, 22). To further characterize insulin sensitivity in these mice, a trait frequently impaired in NAFLD (23, 24), glucose tolerance testing (GTT) was performed at 16 weeks of age. There was lower glucose tolerance in male Arid1a LKO mice on a regular diet, but this difference was not present in the HFD cohorts (Fig. S1D). Histologically, Arid1a LKO HFD mice showed greater amounts of hepatocyte steatosis as compared to their WT counterparts. Sirius Red staining was also increased in these mice, indicating greater levels of fibrosis reflective of later-stage fatty liver disease (Fig. 2E). NAS scoring in these mice showed significantly higher levels of lobular inflammation and hepatocellular ballooning (Fig. 2F). qPCR analysis of genes reflective of inflammation and fibrosis were elevated in Arid1a LKO HFD mice, including Cd11c and Cd68, markers for dendritic cells and monocytes, and a-Sma, and Col1a2, signs of increased myofibroblast activation and collagen deposition (Fig. S1E). Overall, these data also suggested that Arid1a deficiency within the liver increased susceptibility to diet-induced fatty liver disease.
Figure 2. Arid1a LKO mice were more susceptible to nutritional challenges.
A. Study design for the high fat diet (HFD) trial. Diet was started at 6 weeks of age, with glucose tolerance test (GTT) performed at 16 weeks, body composition (BC) measurement at 17 weeks, and mouse sacrifice at 18 weeks.
B. Comparison of body parameters in male and female cohorts, including final body weight and LW/BW ratio (n = 5,8,5,5 for males and 8,6,6,6 for females).
C. Notable serum values in the male cohort. (n = 5,8,5,5).
D. Hepatic triglycerides and cholesterol from the male cohort expressed as milligrams per gram of tissue. (n = 5,5,6,3).
E. H&E and Sirius Red imaging comparing WT and LKO mice. Fatty infiltration is markedly increased within the LKO mice, especially on HFD. Sirius Red staining, reflective of fibrosis, is modestly increased within LKO mice.
F. NAS scores for WT and LKO mice (n=5,5,8,5)
To examine if the relationship between SWI/SNF expression and fatty liver disease was also true in humans, we analyzed a publically available transcriptomic data set of patients with NASH versus healthy controls (GSE37031) (25). Intriguingly, multiple SWI/SNF components, including ARID1A, showed decreased expression in NASH patients (Fig. S2A). We correlated this data with expression data from human liver tissue obtained from transplant donors with and without steatosis. qPCR in these tissues showed a trend of decreased ARID1A expression in conjunction with elevation of genes related to fatty acid synthesis, suggesting that alterations in ARID1A expression may impact NAFLD development in humans (Fig. S2B).
Arid1a deletion leads to differential expression of lipid homeostasis genes
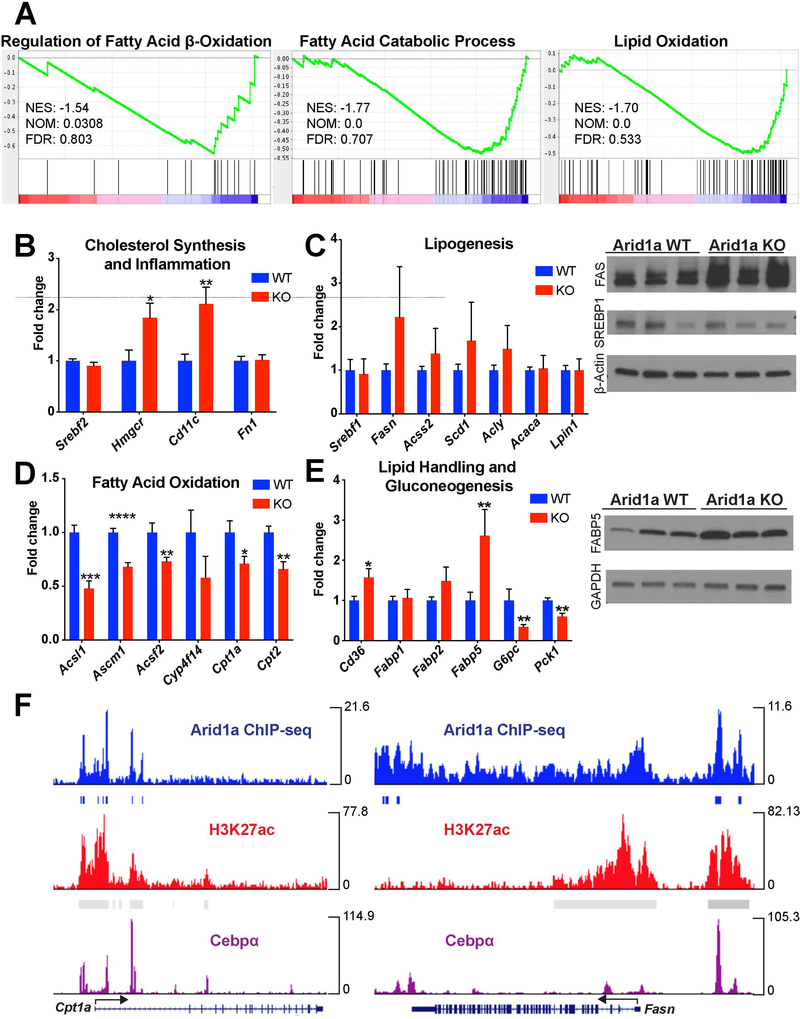
As ARID1A is a known DNA-binding component of the SWI/SNF chromatin remodeling complex, we wondered if ARID1A was exhibiting its effects on hepatic steatosis through epigenetic or transcriptional regulation of downstream genes. To explore this, we analyzed the transcriptome from WT and Arid1a LKO livers using RNA-seq. Gene set enrichment analysis (GSEA) identified significant changes in several key pathways related to lipid metabolism (Fig. 3A). Notably, genes relating to cholesterol homeostasis, lipid handling, and fatty acid metabolism were significantly altered. We examined mRNA expression of 23 genes within these pathways and confirmed significant changes in 11 (Fig. 3B-E). Most notably, genes in the fatty acid beta-oxidation pathway were significant downregulated, including Cpt1a and Cpt2, which are responsible for transporting long chain fatty acids into the mitochondrial matrix, and Acsl1, Acsm1, and Acsf2, all of which encode enzymes that activate fatty acids of differing lengths for beta-oxidation. There was also a trending increase in genes related to de novo lipogenesis, including Fasn, Scd1, and Acss2, as well as increases in genes governing fatty acid handling, cholesterol synthesis, inflammation, and fibrosis. Protein expression validated increases in FABP5 and FAS without corresponding increases in SREBP1 (Fig. 3C,E). Overall, this supports the idea that ARID1A and SWI/SNF impact both lipogenesis and fatty acid oxidation, and that imbalances in these two processes may lead to steatosis and ultimately NASH.
Figure 3. ARID1A bound promoter sequences of many lipid homeostasis genes and regulated their expression.
A. Representative gene sets affected in LKO mice without HFD stimulation. Several pathways relating to lipid handling are significantly affected.
B. qPCR for genes in pathways related to lipid handling. 11 of the 23 genes show significantly altered expression levels in LKO livers (n = 9), including cholesterol synthesis and inflammation,
C. Lipogenesis (with associated Western blotting),
D. Fatty acid oxidation,
E. And lipid handling and gluconeogenesis (with associated Western blotting).
F. Representative ChIP-seq data showing Arid1a binding peaks at promoter sequences of genes seen to be differentially expressed in LKO livers. These peaks coincide with H3K4me3, H3K27ac, and C/ebpα binding peaks at the same location.
Strikingly, genes involved in gluconeogenesis were downregulated in Arid1a LKO mice (Fig. 3E), an interesting finding given that gluconeogenesis is not suppressed in settings of insulin resistance (26). However, this coincides well with our previous findings that showed normal blood glucose levels within Arid1a LKO mice, even in the setting of advanced NAFLD or NASH (Fig. 1E,F).
To assess the affect of ARID1A in human cells, we tested expression levels of these genes in two HCC cell lines with and without Arid1a knockdown. Compared to shGFP control cells, ARID1A knockdown HepG2 cells showed upregulation of fatty acid binding proteins (FABP1,2,5) as well as SREBF1, ACACA, and HMGCR, and downregulation of CPT1A (Fig. S3A). Huh-7 cells had upregulation of SCD1 and ACSS2 and decreased expression of ACSM1 (Fig. S3B). Western blotting in HepG2 also showed an increase in FAS levels (Fig. S3C).
To better characterize the nature of ARID1A’s interaction with the loci of affected genes, we analyzed previously generated in vivo ChIP-seq data for the targets of ARID1A within the liver (13). Of the 11 genes previously confirmed to have significantly altered expression, 9 had ARID1A binding peaks near their transcriptional start sites, including Cpt1a, Cpt2, and Acsl1 (Fig. 3F). Lipogenic genes also showed ARID1A binding sites near their transcriptional start sites, including Fasn, Acaca, and Lpin1. These binding peaks were associated with C/ebpα transcription factor binding peaks as well as increased H3K27ac and H3K4me3, marks of active cis-regulatory elements. A similar analysis of publically available ARID1A ChIP-seq data in HepG2 cells showed significant peaks upstream in all six of the differentially regulated genes (Fig. S3D) (27). The overlap between ARID1A and C/ebpα binding peaks within the regulatory elements of these genes suggest that ARID1A exerts direct rather than indirect effects on transcriptional access, potentially through nucleosome repositioning.
Loss of Arid1a and Pten synergistically contribute to fatty liver pathogenesis.
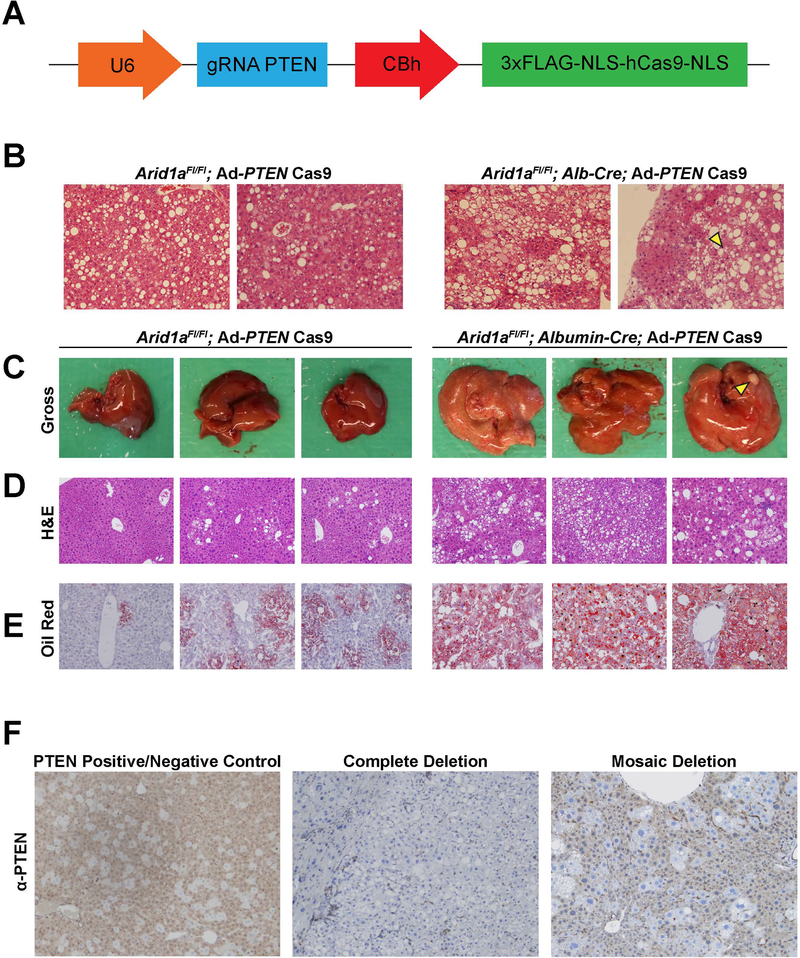
A challenge for the study of NASH pathogenesis is the fact that mouse models generally develop fatty liver disease very slowly, thus preventing efficient assessment of disease progression factors and therapeutic strategies. Pten is known to repress fatty liver formation, and Pten deletion leads to steatosis and HCC within 78 weeks or 1.4 years (28). Previous studies have shown that ARID1A and PTEN mutations frequently coexist in human cancers and that loss of ARID1A sensitizes cancer cells to inhibition of the PI3K/Akt pathway, which PTEN regulates (29, 30). To determine if Arid1a loss synergizes with Pten loss in steatotic phenotypes, we employed an adenovirus carrying Cas9 and a guide strand targeting Pten (Fig. 4A). We then introduced this Cas9 virus via intravenous injection of WT and Arid1a LKO mice at 6 weeks of age. By 5 months of age, the double KO mice had developed advanced steatosis and occasional dysplasia compared to their single KO counterparts (Fig. 4B). By 8 months of age, the livers of these mice showed greatly increased steatosis compared to their single Arid1a LKO counterparts, and included the development of rare HCCs (Fig. 4C,D). Oil Red O staining for lipid droplets was also increased in the double KO livers compared to single knockouts, indicating greater steatosis (Fig. 4E). Immunostaining within these livers showed complete lack of PTEN in most cells in 5 out of the 6 mice tested; the remaining mouse liver showed mosaic deletion comprising roughly 50% of the tissue. (Fig. 4F). This accelerated NASH model facilitated the testing of novel therapeutic approaches.
Figure 4. Adenovirus-mediated deletion of Pten accelerated fatty liver development within Arid1a LKO mice.
A. Schematic illustrating the sgPTEN-Cas9 adenovirus used to delete PTEN in the double KO mouse model.
B. Representative H&E images from PTEN KO and double KO mice at 5 months of age.
C. Representative gross livers from PTEN KO and double KO mice at 8 months of age. Yellow arrowhead indicates tumor.
D. Representative H&E images from mice at 8 months of age.
E. Representative Oil Red O stained images from mice at 8 months of age.
F. Representative IHC staining for mice at 8 months of age. Control tissue is mosaic for PTEN deletion. 5 of the 6 mice imaged showed full deletion (second image), while one had partial deletion (third image).
SREBP inhibition delayed fatty liver caused by Arid1a and Pten loss.
Given the fact lipogenesis is promoted in the Arid1a deficient state, we wanted to determine if this might be a therapeutic target for NASH progression within the more aggressive Arid1a/Pten double KO model. To that end, we treated double KO mice with CAT-2003, an orally bioavailable small molecule that inhibits SREBP maturation and activation of SREBP target genes (31). SREBP-1c is an attractive target for this double-KO model since it is known to be a master regulator of enzymes in the de novo lipogenesis pathway (32). In order to test CAT-2003 with two treatment paradigms, we established prophylactic and therapeutic cohorts using single Pten KO and double KO mice. The prophylactic cohort began receiving CAT-2003 immediately after adenovirus injection at 7 weeks, while the therapeutic cohort began receiving CAT-2003 at 22 weeks of age (Fig. 5A).
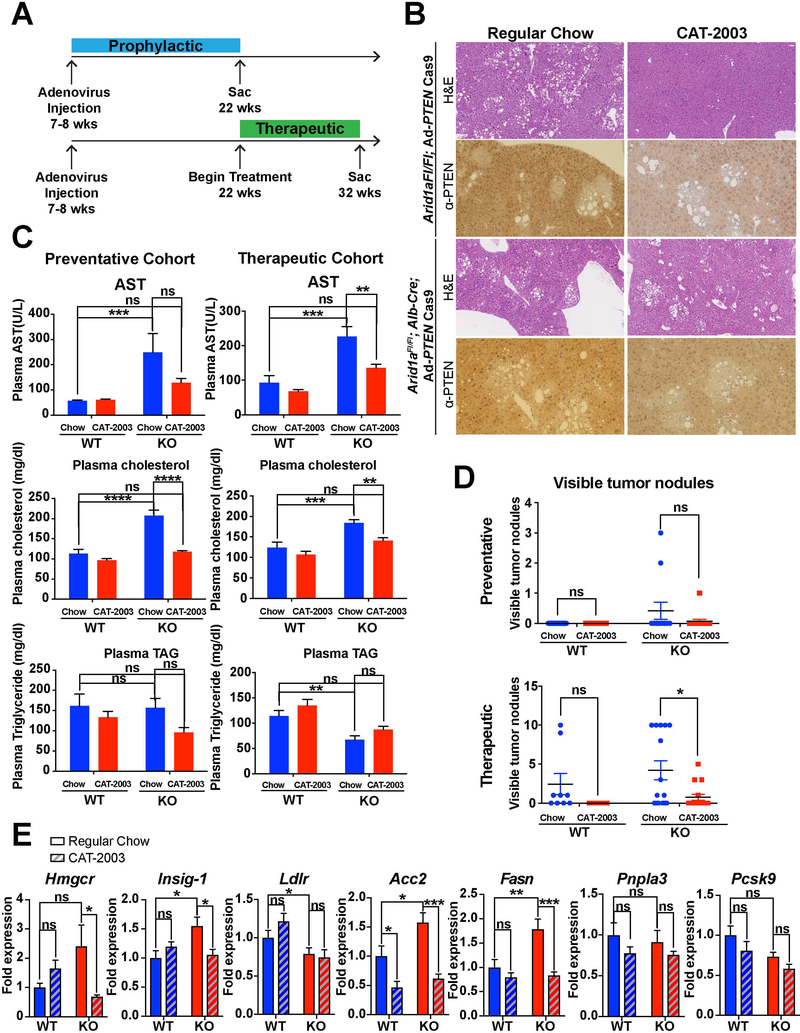
Figure 5. CAT-2003, a small molecular inhibitor of SREBP-1c, delayed progression of fatty liver disease in the Arid1a; Pten double KO model.
A. Treatment schedule for both the preventative and therapeutic cohorts. PTEN injection was given at 7 weeks of age, followed by sacrifice at 22 weeks for prevention and 32 weeks for therapeutic. Treatment was begun at 7 weeks of age for prevention and 22 weeks of age for the therapeutic cohort.
B. Representative H&E and Pten-IHC for mice within the therapeutic cohort.
C. Notable serum markers of fatty liver disease in both the preventative and therapeutic cohorts (n = 8).
D. Comparisons of visible surface tumors in both the preventative and therapeutic cohorts (n = 19,20,12,15 for the preventative cohort and 9,7,15,15 for the therapeutic cohort).
E. SREBP-1c target expression levels measured via qPCR in the prophylactic cohort (n = 8).
Histologically, the amount of steatosis was variable between and within treatment groups, with fatty areas of tissue distributed in clusters ranging from scattered nodules to a majority of the tissue. PTEN IHC within this tissue revealed that the areas of fat infiltration overlapped almost entirely with areas of PTEN absence, suggesting that cells successfully infected with adenovirus were able to clonally expand (Fig. 5B). There were no clear signs of tumor development apparent on these sections, but much of the Pten null fatty tissue appeared dysplastic.
Body parameters including body weight, liver weight, liver weight/body weight ratio, and waist circumference were unaffected by administration of CAT-2003 in both the prophylactic and therapeutic cohorts (Fig. S4). However, serum markers of steatohepatitis, most notably serum cholesterol, were significantly decreased in both cohorts (Fig. 5C). We measured expression levels of known downstream targets of SREBP-1c via qPCR within both cohorts. Arid1a LKO mice within the prophylactic cohort treated with CAT-2003 had decreased expression of many of these targets, including Hmgcr, Fasn, Insig-1, and Acc2; these differences were not seen within the therapeutic cohort (Fig. 5E). Within the therapeutic cohort, however, there was a significant decrease in the amount of surface tumors observed in the double KO mice treated with CAT-2003 (Fig. 5D).
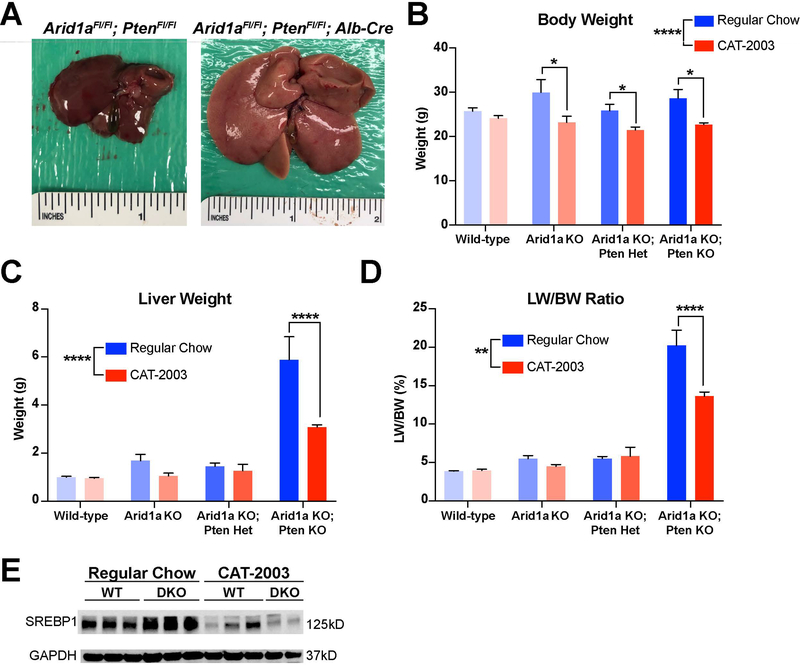
To confirm the veracity of the Cas9 Pten deletion model, we also generated a fully penetrant Arid1a/Pten double knockout model using Alb-Cre to delete both genes specifically within the liver. These mice developed signs of fatty liver even more rapidly. Deletion of both alleles from birth showed a marked enlargement and paling of the liver (Fig 6A). Mice were given either regular chow or CAT-2003 for 8 weeks beginning at 6 weeks of age. Administration of CAT-2003 significantly decreased body weight, liver weight, and LW/BW within the DKO cohorts (Fig. 6B-D). Importantly, western blotting in these tissues showed that SREBP1 is increased in DKO vs. control livers, an increase that is then reversed in the context of livers treated with CAT-2003, indicating that the drug is successfully engaging in the intended target within hepatocytes (Fig. 6E).
Figure 6. A fully penetrant Arid1a; Pten double KO mouse model shows increased response to SREBP-1c inhibition.
A. Representative gross images of wild-type and Arid1a/Pten double KO livers.
B. Final body weights of mice given either regular chow or CAT-2003 diet (n = 6,8; 5,5; 9,8; 4,5).
C. Liver weights of mice given either regular chow or CAT-2003 diet.
D. Liver weight/body weight ratio of mice given either regular chow or CAT-2003 diet.
E. Western blot for SREBP1 in WT and double KO mice on both regular chow and CAT-2003 diet.
Discussion
NAFLD and NASH continue to rise in prevalence in both the United States and worldwide, and it will likely continue to represent a growing proportion of chronic and end-stage liver disease in the coming decades. Despite this, its pathogenesis is still poorly understood, and the factors that determine which patients will progress and which will stagnate remain unclear. Here, we report an epigenetic role for the regulation of lipid processing via the chromatin remodeling protein ARID1A. We observe that Arid1a loss in mice drives the development of fatty liver at baseline and potentiates the liver’s response to nutritional challenges via HFD. Our work suggests that ARID1A may be exerting its effect through direct regulation of target genes in both the lipogenic and fatty acid oxidation pathways by binding promoter sequences and altering accessibility to transcriptional machinery.
The Arid1a LKO model is especially intriguing given its apparent insulin sensitivity and normal serum triglyceride levels, two parameters that are classically disturbed in metabolic fatty liver. However, a growing body of research is demonstrating that certain genetic factors, such as PNPLA3, may induce a type of fatty liver disease that differs from the normal metabolic syndrome (33, 34). In particular, patients with PNPLA3-mediated fatty liver disease show a lack of insulin resistance and normal serum triglyceride levels due to an altered lipidomic landscape within the liver with fewer ceramides, and a failure to export lipids synthesized within the liver to the bloodstream (35). Given the similarities, it is possible that there is some mechanistic overlap between this genetic model of NAFLD and the Arid1a loss-driven model.
One of the issues in studying fatty liver disease is that it often develops very slowly, taking months or even years to achieve NASH or cirrhosis in mouse models. Combining Pten deletion, a well-established tumor suppressor gene whose loss drives steatohepatitis via the PI3K/Akt signaling pathway (36), with Arid1a deletion shows the potential for a model of severe fatty liver disease that results in tumor development within 6 months, a marked acceleration when compared to other fatty liver models.
We used this accelerated model to explore how a small molecule inhibitor of SREBP-1c might impact both fatty liver progression and tumor development. In the treated mice, we see clear improvements in serum markers of disease, including AST, ALT, and cholesterol. The impact of tumor development was modest, which may be due to both the relatively young age of the cohorts as well as the incomplete deletion of Pten observed within these livers. We do observe considerable improvement in the treated cohorts, suggesting that loss of ARID1A may be at least partially rescued by inhibition of known downstream targets, lending further credence to ARID1A’s role in the maintenance of lipid homeostasis.
Supplementary Material
Acknowledgments
Financial Support
A.M is supported by a Howard Hughes Medical Institute Medical Fellowship and a NIH T32 Training Grant (5 T32 CA 124334–12). X.L. is supported by Cancer Prevention and Research Institute of Texas (CPRIT RP150596). L.W. is supported by the National Natural Science Foundation of China (grant 81670592); the Science and Technology Program of Guangzhou, China (grant 201804020075); and the Fundamental Research Funds for the Central Universities (grant 17ykjc09). H.Z. is supported by a NIH/NIDDK R01 grant (R01DK111588), a Burroughs Welcome Career Award for Medical Scientists, a CPRIT New Investigator grant (R1209), a CPRIT Early Translation grant (DP150077), and a AACR/Stand Up to Cancer Innovative Research Grant (SU2C-AACR-IRG-10–16).
List of Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- HCC
hepatocellular carcinoma
- MeCP2
methyl CpG binding protein 2
- PRC1
polycomb repressive complex 1
- PPARγ
peroxisome proliferator-activated receptor gamma
- MLL4
mixed lineage leukemia 4
- SWI/SNF
SWItch/sucrose non-fermentable
- BRM
brahma
- BRG1
brahma-related gene 1
- ARID1A
AT-rich interactive domain-containing protein 1A
- TNF-α
tumor necrosis factor alpha
- IL-6
interleukin 6
- PTEN
phosphatase and tensin homolog
- PBS
phosphate buffered saline
- IPGTT
intraperitoneal glucose tolerance testing
- GSEA
gene set enrichment analysis
- ChIP-seq
chromatin immunoprecipitation sequencing
- LKO
liver knockout
- NAS
NAFLD activity score
- HFD
high fat diet
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- WT
wild-type
- SREBP-1c
sterol regulatory element-binding protein 1c
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- DKO
double knockout
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–285. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9:524–530 e521; quiz e560. [DOI] [PubMed] [Google Scholar]
- 4.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol 2016;11:451–496. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, Garrett ME, et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeybel M, Hardy T, Robinson SM, Fox C, Anstee QM, Ness T, Masson S, et al. Differential DNA methylation of genes involved in fibrosis progression in non-alcoholic fatty liver disease and alcoholic liver disease. Clin Epigenetics 2015;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 2010;138:705–714, 714.e701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Gomez G, Gray S, Vidal-Puig A. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1). Public Health Nutr 2007;10:1132–1137. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Chan CC, Kwon OS, Liu S, McGhee J, Stimpson SA, Chen LZ, et al. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2006;291:G902–911. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Kim J, Kwon JS, Sandhu J, Tontonoz P, Lee SK, Lee S, et al. Critical Roles of the Histone Methyltransferase MLL4/KMT2D in Murine Hepatic Steatosis Directed by ABL1 and PPARgamma2. Cell Rep 2016;17:1671–1682. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol 2010;102:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler RL, Brennan J, Schisler JC, Serber D, Patterson C, Magnuson T. ARID1a-DNA interactions are required for promoter occupancy by SWI/SNF. Mol Cell Biol 2013;33:265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Chuang JC, Kanchwala M, Wu L, Celen C, Li L, Liang H, et al. Suppression of the SWI/SNF Component Arid1a Promotes Mammalian Regeneration. Cell Stem Cell 2016;18:456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Wang SC, Wei Y, Luo X, Jia Y, Li L, Gopal P, et al. Arid1a Has Context-Dependent Oncogenic and Tumor Suppressor Functions in Liver Cancer. Cancer Cell 2017;32:574–589.e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang JZ, Li C, Liu XY, Hu TT, Fan ZS, Han ZG. Hepatocyte-Specific Arid1a Deficiency Initiates Mouse Steatohepatitis and Hepatocellular Carcinoma. PLoS One 2015;10:e0143042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A 2008;105:6656–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 18.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, Cai W, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 2014;514:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caballero F, Fernandez A, De Lacy AM, Fernandez-Checa JC, Caballeria J, Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol 2009;50:789–796. [DOI] [PubMed] [Google Scholar]
- 22.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007;46:1081–1090. [DOI] [PubMed] [Google Scholar]
- 23.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004;114:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angulo P Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–1231. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Vicario C, Gonzalez-Periz A, Rius B, Moran-Salvador E, Garcia-Alonso V, Lozano JJ, Bataller R, et al. Molecular interplay between Delta5/Delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 2014;63:344–355. [DOI] [PubMed] [Google Scholar]
- 26.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000;6:87–97. [PubMed] [Google Scholar]
- 27.Raab JR, Resnick S, Magnuson T. Genome-Wide Transcriptional Regulation Mediated by Biochemically Distinct SWI/SNF Complexes. PLoS Genet 2015;11:e1005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest 2004;113:1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosse T, ter Haar NT, Seeber LM, v Diest PJ, Hes FJ, Vasen HF, Nout RA, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod Pathol 2013;26:1525–1535. [DOI] [PubMed] [Google Scholar]
- 30.Samartzis EP, Gutsche K, Dedes KJ, Fink D, Stucki M, Imesch P. Loss of ARID1A expression sensitizes cancer cells to PI3K- and AKT-inhibition. Oncotarget 2014;5:5295–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmer M, Bista P, Benson EL, Lee DY, Liu F, Picarella D, Vega RB, et al. CAT-2003: A novel sterol regulatory element-binding protein inhibitor that reduces steatohepatitis, plasma lipids, and atherosclerosis in apolipoprotein E*3-Leiden mice. Hepatol Commun 2017;1:311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton JD, Shimomura I. Sterol regulatory element-binding proteins: activators of cholesterol and fatty acid biosynthesis. Curr Opin Lipidol 1999;10:143–150. [DOI] [PubMed] [Google Scholar]
- 33.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yki-Jarvinen H, Luukkonen PK. Heterogeneity of non-alcoholic fatty liver disease. Liver Int 2015;35:2498–2500. [DOI] [PubMed] [Google Scholar]
- 35.Luukkonen PK, Zhou Y, Sadevirta S, Leivonen M, Arola J, Oresic M, Hyotylainen T, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 2016;64:1167–1175. [DOI] [PubMed] [Google Scholar]
- 36.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998;273:13375–13378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.