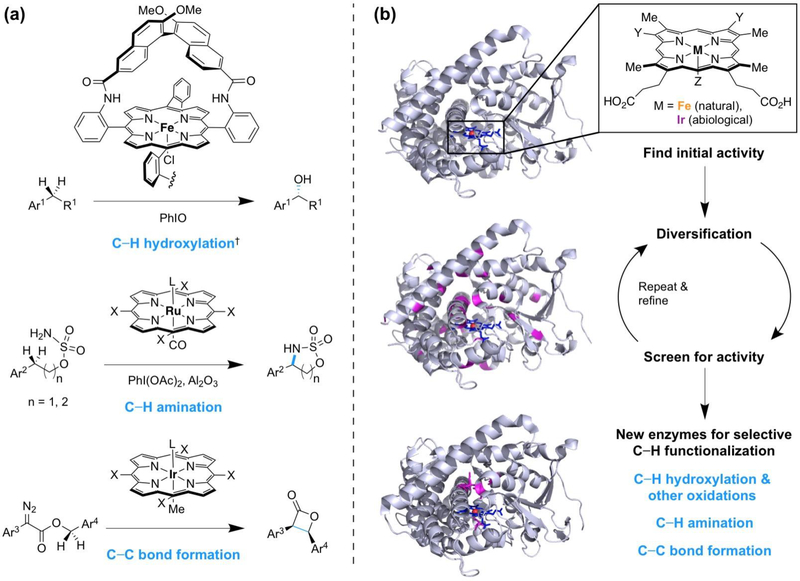
Figure 1.
Porphyrin is a versatile scaffold for C—H bond functionalization. (a) Porphyrin-based transition metal catalysts which functionalize C—H bonds. Examples are from ref. [4–6]. X, (1S,4R,5R,8S)-1,2,3,4,5,6,7,8-octahydro-1,4:5,8-dimethanoanthracene-9-yl. †Corresponding ketones were also formed; ketone formation is not due to further oxidation of alcohol products [4]. (b) Proteins which contain a porphyrin group have been engineered by directed evolution to perform C—H oxidation reactions with increased activity or tailored site-selectivity, C—H amination, and carbene C—H insertion. Structural models are Bacillus megaterium cytochrome P450BM3 (PDB 1JPZ, top and middle) and an engineered C—H amination enzyme derived from P450BM3 (PDB 5UCW, bottom); Y, vinyl or ethyl; Z, amino acid or organic functional group.