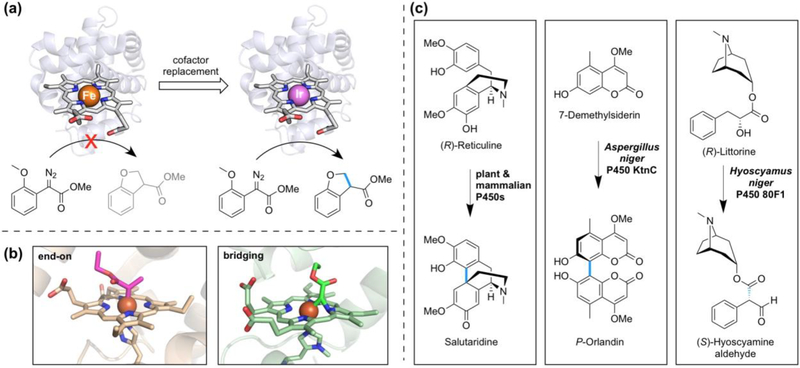
Figure 4.
C—H to C—C bond transformations catalyzed by artificial metalloenzymes and cytochromes P450. (a) Replacement of heme in myoglobin with Ir(Me)-mesoporphyrin IX results in an artificial metalloenzyme which catalyzes carbene C—H insertion, a reaction that the iron-based enzyme does not catalyze [47••]. Cartoons were created using PDB 1MBN; porphyrin cofactors have been enlarged (not to scale). (b) The IPC intermediate has been captured by X-ray crystallography in two poses, end-on (left, PDB 6CUN) and Fe–C– N(pyrrole) bridging (right, PDB 6G5B), in engineered cytochrome c and myoglobin carbene transferases, respectively [51•, 52•]. (c) P450 catalyzed C—C bond forming reactions in natural product biosynthesis. Representative examples are from ref. [10], [56], and [57] (left to right).