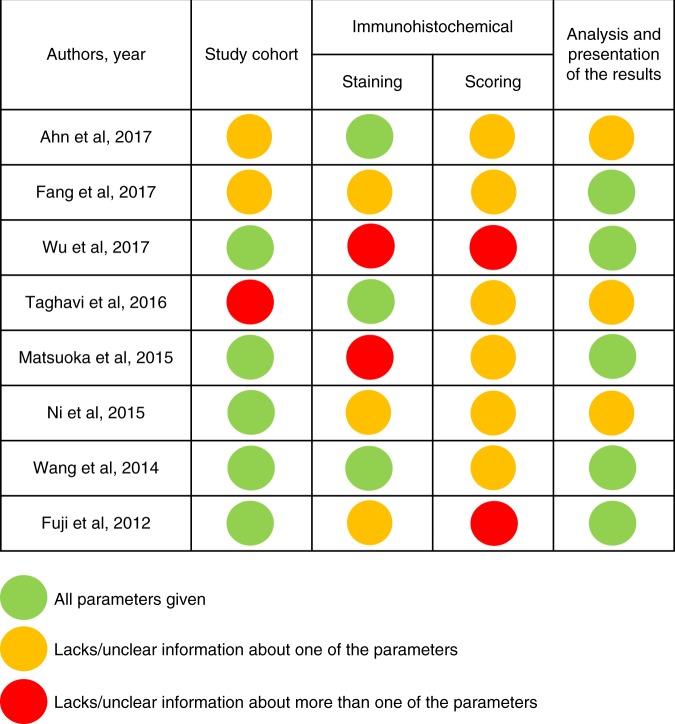
Fig. 3.
Assessment of the reporting quality of the studies included in the meta-analysis. The reporting of the following parameters was evaluated: Study cohort, (1) number of patients, (2) tumour size/stage, (3) chemotherapy/radiation of tissue prior to surgery; Immunohistochemical staining, (1) antibody clone/product number, (2) immunohistochemistry procedures, (3) positive and negative controls; scoring (1) number of observers, (2) clear scoring criteria, (3) inter/intra-observer variability; Analysis and presentation of the results, (1) survival endpoint analysed, (2) direction of effect on survival in Kaplan–Meier plot, (3) estimated effects with confidence intervals for the marker and, at least for the final model, all other variables in the model