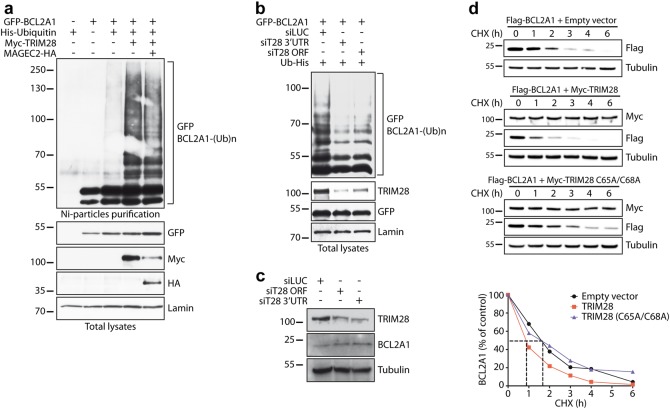
Fig. 2.
TRIM28 regulates the ubiquitination and degradation of BCL2A1. a HEK293T cells were transfected with GFP-BCL2A1, Myc-TRIM28, and MAGEC2-HA constructs as indicated, together with His-tagged ubiquitin (Ub-His) for 18 h. Then cells were incubated with MG132 for 6 h. Total ubiquitinated proteins were purified using nickel beads and analyzed by western blot using anti-GFP antibody to detect poly-ubiquitinated forms of BCL2A1. Initial total lysates were analyzed for the expression of the different proteins by immunoblot. b HEK293T cells were first transfected with two different siRNAs to inhibit TRIM28 expression. 24 h later, cells were transfected with GFP-BCL2A1 and Ub-His for one additional day. Then, cells were treated and cell lysates were analyzed. c SK-MEL-28 cells were transfected with two different siRNAs for two consecutive days to inhibit TRIM28 expression. Cells were collected 48 h after the first siRNA transfection. The efficiency of TRIM28 silencing and its effect on the protein level of endogenous BCL2A1 were assessed by immunoblot. d HEK293T cells were co-transfected with Flag-tagged BCL2A1 and Myc-tagged TRIM28 or an inactive RING mutant C65/68A of TRIM28 for 24 h. Transfected cells were treated with the protein synthesis inhibitor cycloheximide (CHX, 10 μg/ml) for increasing times as indicated. Total protein extracts were analyzed by immunoblot. The protein level of Flag-BCLA1 was followed with time using anti-Flag antibody in order to measure its half-life. Anti-Myc antibody was used to verify equal expression of TRIM28 and anti-tubulin antibody to assess equal loading. Data shown are representative of three independent experiments. BCL2A1 protein level was quantified by densitometry and was expressed as a percentage of the value measured at time zero for each of the three conditions