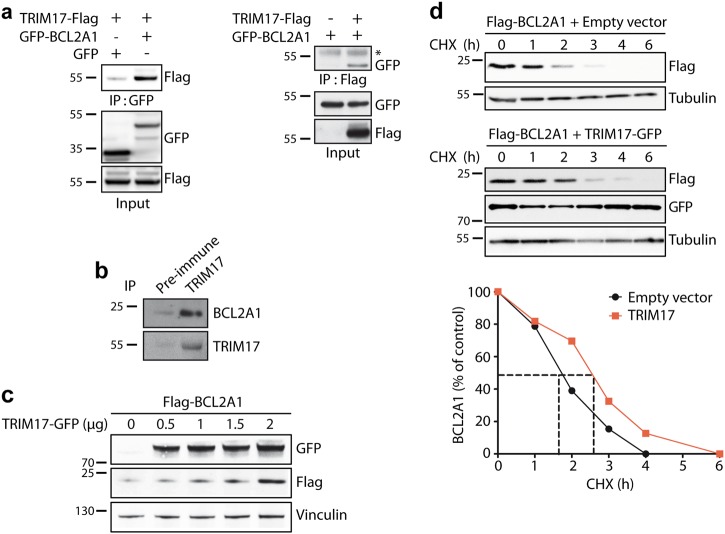
Fig. 3.
TRIM17 induces the stabilization of BCL2A1 protein. a HEK293Tcells were transfected with GFP-tagged BCL2A1 and TRIM17-Flag as indicated. Cell lysates were subjected to immunoprecipitation with GFP-Trap beads (left) or Flag-beads (right), and the presence of TRIM17 or BCL2A1 was detected by western blot using anti-Flag or anti-GFP antibodies respectively (*) shows IgG heavy chains. b SK-MEL-28 protein extract was subjected to immunoprecipitation using an anti-TRIM17 antibody or the corresponding pre-immune serum as an negative control, as indicated. BCL2A1 and TRIM17 proteins were detected in the immunoprecipitate by western blot using specific antibodies. c HEK293T cells were transfected with FLAG-BCL2A1 and increasing amounts of TRIM17-GFP vectors for 48 h. Total protein extracts were subjected to immunoblot analyses using the indicated antibodies. d HEK293T cells were transfected with Flag-tagged BCL2A1 in the presence or the absence of GFP-tagged TRIM17 for 24 h. Transfected cells were treated with cycloheximide (CHX, 10 μg/ml) for the indicated time periods. Total protein extracts were analyzed by immunoblot. The protein level of Flag-BCLA1 was followed using anti-Flag antibody. Anti-GFP antibody was used to verify equal expression of TRIM17 and anti-tubulin antibody to assess equal loading. Data shown are representative of three independent experiments. BCL2A1 protein level was quantified by densitometry and was expressed as a percentage of the value measured at time zero for each of the two conditions